dimethyl 2-(3-oxo-1-phenylpropyl)malonate | 136917-91-4
中文名称
——
中文别名
——
英文名称
dimethyl 2-(3-oxo-1-phenylpropyl)malonate
英文别名
methyl 2-methoxycarbonyl-3-phenyl-5-oxopentanoate;Dimethyl 2-(3-oxo-1-phenylpropyl)propanedioate;dimethyl 2-(3-oxo-1-phenylpropyl)propanedioate
CAS
136917-91-4
化学式
C14H16O5
mdl
——
分子量
264.278
InChiKey
WNDVIPBSSNSAQS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):1.4
-
重原子数:19
-
可旋转键数:8
-
环数:1.0
-
sp3杂化的碳原子比例:0.36
-
拓扑面积:69.7
-
氢给体数:0
-
氢受体数:5
反应信息
-
作为反应物:参考文献:名称:A comparison of the benzylic and the allylic group as a donor in the formal [4+2] cycloaddition to tetrahydropyrans using donor-acceptor cyclobutanes摘要:The allyl group was shown to be preferred over the benzyl group as a donor in the formal [4+2]-cyclo-addition reaction between a donor-acceptor cyclobutane and various aldehydes to give tetrahydropyrans. (C) 2017 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tetlet.2017.06.046
-
作为产物:参考文献:名称:The Michael addition of dimethyl malonate to α,β-unsaturated aldehydes catalysed by proline lithium salt摘要:在存在10 mol%的脯氨酸锂盐的条件下,二甲基丙二酸酯与α,β-不饱和醛反应生成了高收率的迈克尔加成产物。DOI:10.1039/c39910001088
文献信息
-
Polystyrene-Supported Diarylprolinol Ethers as Highly Efficient Organocatalysts for Michael-Type Reactions作者:Esther Alza、Sonia Sayalero、Pinar Kasaplar、Diana Almaşi、Miquel A. PericàsDOI:10.1002/chem.201101730日期:2011.10.4addition of aldehydes to nitroolefins and of malonates or nitromethane to α,β‐unsaturated aldehydes. The combination of the catalytic unit, the triazole linker, and the polymeric matrix provides unprecedented substrate selectivity, in favor of linear, short‐chain aldehydes, when the organocatalyzed reaction proceeds by an enamine mechanism. High versatility is noted in reactions that proceed via an iminium锚固在聚苯乙烯树脂上的α,α-二苯基脯氨醇甲基和三甲基甲硅烷基醚是通过铜催化的叠氮化物-炔烃环加成反应(CuAAC)制备的。O显示的催化活性和对映选择性三甲基甲硅烷基衍生物与最著名的均相催化剂表现出的可比性相当,后者可将醛加成到硝基烯烃中,将丙二酸酯或硝基甲烷加成到α,β-不饱和醛上。当有机催化的反应通过烯胺机制进行时,催化单元,三唑连接基和聚合物基体的组合可提供前所未有的底物选择性,有利于线性短链醛。通过亚胺离子中间体进行的反应具有很高的通用性。还通过不对称迈克尔加成反应评估了聚苯乙烯负载的α,α-二苯基脯氨醇甲醚的催化行为。通常,将二芳基脯氨醇醚固定在不溶性聚苯乙烯树脂上的CuAAC具有重要的操作优势,例如高催化活性,
-
Highly Diastereo- and Enantioselective Cascade Synthesis of Bicyclic Lactams in One-Pot作者:Yan Jiang、Luca Deiana、Rana Alimohammadzadeh、Leifeng Liu、Junliang Sun、Armando CórdovaDOI:10.1002/ejoc.201701789日期:2018.3.7A versatile and highly stereoselective synthetic route to functionalized bi- and tricyclic lactams (up to > 20:1 dr and 99 % ee) in one pot from simple starting materials (allylic alcohols, enal ...
-
A Recyclable Chiral 2‐(Triphenylmethyl)pyrrolidine Organocatalyst Anchored to [60]Fullerene作者:Cristian Rosso、Marco G. Emma、Ada Martinelli、Marco Lombardo、Arianna Quintavalla、Claudio Trombini、Zois Syrgiannis、Maurizio PratoDOI:10.1002/adsc.201900009日期:2019.6.18[60]fullerene via click chemistry provides a highly efficient supported enantioselective organocatalyst, which was successfully exploited in a Michael addition of malonates to cinnamaldehydes, via iminium ion activation. The supported organocatalyst was recycled up to six times, with only a moderate decrease in terms of activity and with no loss in enantioselectivity.
-
An enantio- and diastereoselective approach to indoloquinolizidines in continuous flow作者:Moreshwar B. Chaudhari、Prachi Gupta、Patricia Llanes、Leijie Zhou、Nicola Zanda、Miquel A. PericàsDOI:10.1039/d2ob01462a日期:——short synthesis of indoloquinolizidines. Using this approach, a highly enantioselective, solvent-free and rapid conjugate addition of dimethyl malonate to a diverse family of cinnamaldehydes in continuous flow, allowing the preparation of relevant oxodiesters in multigram amounts has been developed. The obtained Michael adducts have been used to complete an expedient diastereoselective synthesis of indoloquinolizidine
-
Distribution of Catalytic Species as an Indicator To Overcome Reproducibility Problems作者:Xavier Companyó、Jordi BurésDOI:10.1021/jacs.7b05045日期:2017.6.28Irreproducibility is a common issue in catalysis. The ordinary way to minimize it is by ensuring enhanced control over the factors that affect the reaction. When control is insufficient, some parameters can be used as indicators of the reaction performance. Herein we describe the use of the distribution of catalytic species as an indicator to map, track, and fine-tune the performance of catalytic reactions. This indicator is very sensitive and presents a quick response to variations in the reaction conditions. We have applied this new strategy to the conjugate addition of C-nucleophiles to enals via iminium intermediates, consistently achieving maximum turnover frequencies (TOF) regardless of the quality of the starting materials used. In addition, the present method has allowed us to efficiently reduce the catalyst loading to as little as 0.1 mol %, the lowest one described for this kind of reaction.
表征谱图
-
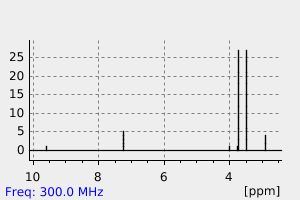
氢谱1HNMR
-
质谱MS
-
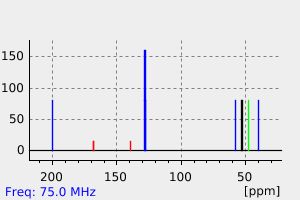
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯