2-乙硫基吩噻嗪 | 46815-10-5
中文名称
2-乙硫基吩噻嗪
中文别名
2-乙硫代吩噻嗪
英文名称
2-(ethylthio)-10H-phenothiazine
英文别名
3-Aethylmercapto-phenothiazin;2-ethylthiophenothiazine;10H-Phenothiazine, 2-(ethylthio)-;2-ethylsulfanyl-10H-phenothiazine
CAS
46815-10-5
化学式
C14H13NS2
mdl
——
分子量
259.396
InChiKey
DMHPUUIDINBWBN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:100 °C
-
沸点:430.5±34.0 °C(Predicted)
-
密度:1.30±0.1 g/cm3(Predicted)
-
保留指数:2750
-
稳定性/保质期:
常规情况下不会分解,也没有任何危险反应。
计算性质
-
辛醇/水分配系数(LogP):5
-
重原子数:17
-
可旋转键数:2
-
环数:3.0
-
sp3杂化的碳原子比例:0.14
-
拓扑面积:62.6
-
氢给体数:1
-
氢受体数:3
安全信息
-
海关编码:2934999090
-
储存条件:密封、阴凉、干燥保存。
SDS
2-Ethylthiophenothiazine Revision number: 5
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 2-Ethylthiophenothiazine
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
No signal word
Signal word
Hazard statements None
None
Precautionary statements:
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 2-Ethylthiophenothiazine
Percent: ....
CAS Number: 46815-10-5
Chemical Formula: C14H13NS2
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
2-Ethylthiophenothiazine
Section 5. FIRE-FIGHTING MEASURES
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust as possible so that workers should not be
Engineering controls:
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: Crystal- Powder
Colour: White - Greyish yellow green
Odour: No data available
pH: No data available
Melting point/freezing point:100°C
No data available
Boiling point/range:
Flash point: No data available
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
No data available
Relative density:
Solubility(ies):
No data available
[Water]
[Other solvents] No data available
2-Ethylthiophenothiazine
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx), Sulfur oxides
products:
Section 11. TOXICOLOGICAL INFORMATION
No data available
Acute Toxicity:
Skin corrosion/irritation: No data available
No data available
Serious eye
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
No data available
Reproductive toxicity:
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
No data available
Fish:
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
No data available
Soil adsorption (Koc):
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
Not listed
UN-No:
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
2-Ethylthiophenothiazine
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 2-Ethylthiophenothiazine
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
No signal word
Signal word
Hazard statements None
None
Precautionary statements:
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 2-Ethylthiophenothiazine
Percent: ....
CAS Number: 46815-10-5
Chemical Formula: C14H13NS2
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
2-Ethylthiophenothiazine
Section 5. FIRE-FIGHTING MEASURES
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust as possible so that workers should not be
Engineering controls:
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: Crystal- Powder
Colour: White - Greyish yellow green
Odour: No data available
pH: No data available
Melting point/freezing point:100°C
No data available
Boiling point/range:
Flash point: No data available
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
No data available
Relative density:
Solubility(ies):
No data available
[Water]
[Other solvents] No data available
2-Ethylthiophenothiazine
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx), Sulfur oxides
products:
Section 11. TOXICOLOGICAL INFORMATION
No data available
Acute Toxicity:
Skin corrosion/irritation: No data available
No data available
Serious eye
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
No data available
Reproductive toxicity:
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
No data available
Fish:
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
No data available
Soil adsorption (Koc):
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
Not listed
UN-No:
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
2-Ethylthiophenothiazine
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3-(乙硫基)-N-苯基苯胺 (3-ethylsulfanyl-phenyl)-phenyl-amine 68083-49-8 C14H15NS 229.346 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 乙巯匹拉嗪 thiethylperazine 1420-55-9 C22H29N3S2 399.624 —— 2-ethylsulfanyl-10-[2-(1-methyl-piperidin-2-yl)-ethyl]-10H-phenothiazine 907-46-0 C22H28N2S2 384.61
反应信息
-
作为反应物:描述:2-乙硫基吩噻嗪 在 sodium hydroxide 、 lithium hexamethyldisilazane 作用下, 以 1,4-二氧六环 、 水 、 N,N-二甲基甲酰胺 、 甲苯 为溶剂, 反应 59.25h, 生成 4-[(2-(ethylthio)-10H-phenothiazin-10-yl)methyl]benzoic acid参考文献:名称:酚噻嗪基苯氧肟酸作为选择性组蛋白脱乙酰基酶6抑制剂的合成及生物学研究。摘要:吩噻嗪系统被确定为有效和选择性组蛋白脱乙酰基酶6(HDAC6)抑制剂的有利封端基团。在这里,我们报告吩噻嗪及其类似物的制备和系统变化,其中吩噻嗪及其类似物含有苯氧肟酸部分作为锌结合基团。我们通过重组HDAC酶分析,通过蛋白质印迹确定细胞中蛋白质的乙酰化水平(微管蛋白与组蛋白乙酰化)以及评估它们对各种癌细胞系的作用,评估了它们选择性抑制HDAC6的能力。构效关系研究表明,将氮原子掺入吩噻嗪骨架中会提高HDAC6的效力和选择性(相对于HDAC1,HDAC4和HDAC8的抑制作用,选择性高500倍以上),通过分子建模和对接研究合理化。通过有效的氮杂吩噻嗪抑制剂与来自Danio rerio HDAC6的催化结构域2的共结晶来确认结合模式。DOI:10.1021/acs.jmedchem.8b01090
-
作为产物:参考文献:名称:氢化催化氢硅烷使用未活化的叔酰胺的选择性还原裂解†摘要:已开发出使用低成本的氢硅烷作为还原剂,对各种未活化的叔酰胺(包括生物活性芳基-吩嗪羧酰胺和具有挑战性的非杂环羰基官能团)进行氢化物催化的还原裂解反应。该新型催化剂体系显示出高效率和排他选择性,在温和条件下以有用的目的提供所需的胺以优异的产率。总的来说,这种无过渡金属的方法可以为目前使用的昂贵的还原剂,高压氢或金属系统提供多种选择,以进行酰胺的选择性还原裂解。DOI:10.1039/c9cy00924h
文献信息
-
Combined KOH/BEt<sub>3</sub> Catalyst for Selective Deaminative Hydroboration of Aromatic Carboxamides for Construction of Luminophores作者:Wubing Yao、Jiali Wang、Aiguo Zhong、Jinshan Li、Jianguo YangDOI:10.1021/acs.orglett.0c03033日期:2020.10.16amides into value-added amine products is a desirable but challenging transformation. Molecules containing iminodibenzyl motifs are prevalent in pharmaceutical molecules and functional materials. Here we established a combined KOH/BEt3 catalyst for deaminative hydroboration of acyl-iminodibenzyl derivatives, including nonheterocyclic carboxamides, to the corresponding amines. This novel transition-metal-free
-
Nucleophilic Aromatic Substitution of Polyfluoroarene to Access Highly Functionalized 10-Phenylphenothiazine Derivatives作者:Kotaro Kikushima、Haruka Koyama、Kazuki Kodama、Toshifumi DohiDOI:10.3390/molecules26051365日期:——polyfluoroarenes with phenothiazine in the presence of a mild base to afford the corresponding 10-phenylphenothiazine (PTH) derivatives. The resulting polyfluoroarene-bearing PTH derivatives were subjected to a second SNAr reaction to generate highly functionalized PTH derivatives with potential applicability as photocatalysts for the reduction of carbon–halogen bonds.
-
Synthesen auf dem Phenothiazin-Gebiet. 2. Mitteilung. N-substituierte Mercaptophenothiazin-Derivate作者:J.-P. Bourquin、G. Schwarb、G. Gamboni、R. Fischer、L. Ruesch、S. Guldimann、V. Theus、E. Schenker、J. RenzDOI:10.1002/hlca.19580410420日期:——The synthesis of a series of pharmacologically interesting N-substituted derivatives of 3-mercaptophenothiazines is described.
-
Te(II)-Catalyzed Cross-Dehydrogenative Phenothiazination of Anilines作者:Pooja Y. Vemuri、Christopher Cremer、Frederic W. PatureauDOI:10.1021/acs.orglett.2c00125日期:2022.3.4Oxidative clicklike reactions are useful for the late-stage functionalization of pharmaceuticals and organic materials. Hence, novel methodologies that enable such transformations are in high demand. Herein we describe a tellurium(II)-catalyzed cross-dehydrogenative phenothiazination (CDP) of aromatic amines. A key feature of this method is a cooperative effect between the phenotellurazine catalyst
-
組成物、硬化膜、カラーフィルタ、カラーフィルタの製造方法、液晶表示装置、発光表示装置、及び熱潜在性ラジカル捕捉剤申请人:大日本印刷株式会社公开号:JP2018024616A公开(公告)日:2018-02-15【課題】高温加熱前後の色差が抑制された組成物、当該組成物を用いて形成された硬化膜、当該組成物を用いて形成されたカラーフィルタ、当該カラーフィルタを有する液晶表示装置及び発光表示装置、並びに加熱により脱保護されてラジカル捕捉剤として機能する新規な熱潜在性ラジカル捕捉剤の提供。【解決手段】(A)一般式(1)で表される化合物と、(B)バインダー成分と、(C)溶剤とを含む、組成物。(QはS又はO;R1及びR2は夫々独立に、ハロゲン原子、アルキル基、アリールアルキル基、アシル基、アルコキシ基、又はアルキルチオ基)【選択図】なし这是一项关于抑制高温加热前后色差的组合物、使用该组合物形成的固化膜、使用该组合物形成的彩色滤光片、具有该彩色滤光片的液晶显示装置和发光显示装置,以及提供一种新的热潜在性自由基捕获剂,其通过加热脱除保护并发挥功能的专利。解决方案包括含有(A)由一般式(1)表示的化合物、(B)粘合剂成分和(C)溶剂的组合物。(其中Q为S或O;R1和R2分别独立地是卤素原子、烷基、芳基烷基、酰基、烷氧基或烷基硫基)。
表征谱图
-
氢谱1HNMR
-
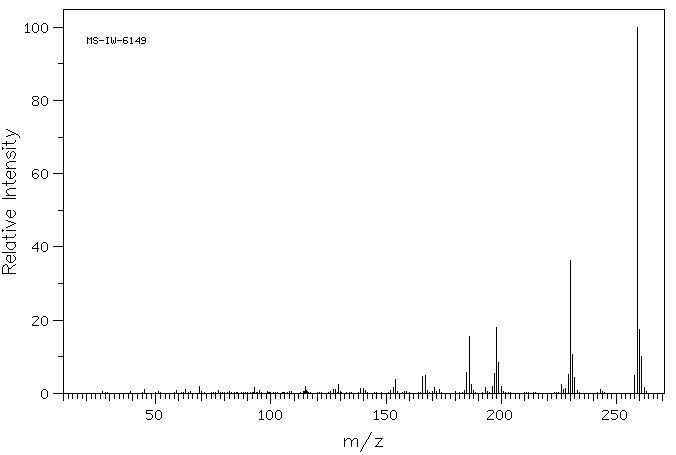
质谱MS
-
碳谱13CNMR
-
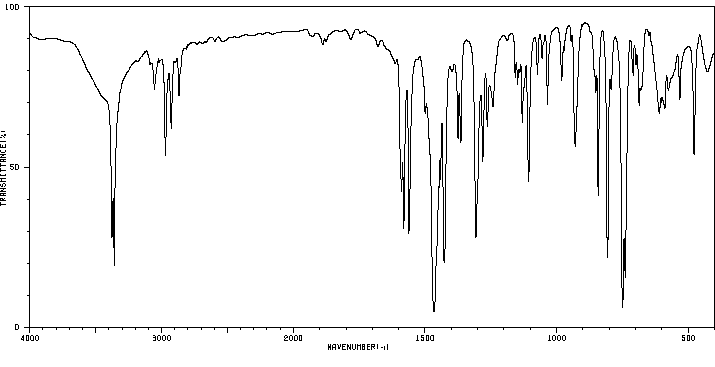
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高氟奋乃静
马来酸甲哌丙嗪
马来酸奋乃静
马来酸乙巯拉嗪
锁匹达新
醋酸奋乃静
醋异丙嗪
酒石酸异丁嗪
还原亚甲蓝
达赛马嗪
螺氯丙嗪
莫雷西嗪亚砜
茶氯酸异丙嗪
苹果酸硫乙拉嗪
苯达莫司汀杂质A
苯甲酸2-(2H-1,4-苯并噻嗪-3-基)酰肼
苯甲酸,4-硝基-2-[[3-(三氟甲基)苯基]氨基]-
苯甲酰基氧基甲基-[3-(2-氯吩噻嗪-10-基)丙基]-二甲基氯化铵
苯并噻嗪-5-氧化
苯并噻嗪-5-正离子,3,7-二(二甲氨基)-4-碘-,氯化
苯并噻嗪,10-(2-(4-丙基-1-哌嗪基)丙基)-
苯并[b]吩噻嗪-12-基(苯基)甲酮
苯并[a]吩噻嗪-5-酮
苯丙嗪
苄酰基无色亚甲基兰
芬诺宁
芬乙嗪
舒多昔康
羟乙哌氟嗪
美索哒嗪
美索丙嗪
美洛昔康钾盐
美洛昔康钠
美洛昔康-d3
美洛昔康
美托奋乃酯
美托哌丙嗪酸
美托哌丙嗪
美托咪嗪-d6
美喹他嗪亚砜
美喹他嗪
磺达嗪
硫堇(劳氏紫)
硫利达嗪杂质A(EP)
硫利达嗪N-氧化物
硫利达嗪-5-亚砜
硫利达嗪
硫代哒嗪-d35-亚砜
硫丙拉嗪
盐酸诺美丙嗪