二溴乙酸乙酯 | 617-33-4
中文名称
二溴乙酸乙酯
中文别名
二溴以乙酸乙酯
英文名称
ethyl dibromoacetate
英文别名
Dibrom-essigsaeure-aethylester;ethyl 2,2-dibromoacetate
CAS
617-33-4
化学式
C4H6Br2O2
mdl
——
分子量
245.898
InChiKey
NIJGVVHCUXNSLL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:77 °C (12 mmHg)
-
密度:1.902
-
闪点:76-78°C/12mm
-
溶解度:易溶于己烷、乙醚、二氯甲烷、丙酮、乙酸乙酯、乙醇。
-
保留指数:1044.9
-
稳定性/保质期:
具有催泪作用,应避免吸入,并在通风橱中进行操作。
计算性质
-
辛醇/水分配系数(LogP):2.2
-
重原子数:8
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
TSCA:Yes
-
危险品标志:Xi
-
安全说明:S26,S37/39
-
危险类别码:R36/37/38
-
危险品运输编号:UN 3265
-
海关编码:2915900090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:将密器密封,存放在主藏器中,并置于阴凉、干燥处。
SDS
| Name: | Ethyl dibromoacetate tech. 83% Material Safety Data Sheet |
| Synonym: | Acetic acid, dibromo-, ethyl este |
| CAS: | 617-33-4 |
Synonym:Acetic acid, dibromo-, ethyl este
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 617-33-4 | Acetic acid, dibromo-, ethyl ester | 83 | 210-510-7 |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.Corrosive.
Potential Health Effects
Eye:
Causes eye burns. May cause chemical conjunctivitis and corneal damage.
Skin:
May cause skin irritation. May cause skin rash (in milder cases), and cold and clammy skin with cyanosis or pale color.
Ingestion:
May cause irritation of the digestive tract. May cause systemic effects.
Inhalation:
Aspiration may lead to pulmonary edema. May cause systemic effects.
Chronic:
Effects may be delayed.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately. Do NOT allow victim to rub eyes or keep eyes closed.
Extensive irrigation with water is required (at least 30 minutes).
Skin:
Get medical aid immediately. Immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid immediately. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Get medical aid immediately. Remove from exposure and move to fresh air immediately. If breathing is difficult, give oxygen. Do not use mouth-to-mouth resuscitation if victim ingested or inhaled the substance; induce artificial respiration with the aid of a pocket mask equipped with a one-way valve or other proper respiratory medical device.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Clean up spills immediately, observing precautions in the Protective Equipment section. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Do not get in eyes, on skin, or on clothing. Do not ingest or inhale. Use only in a chemical fume hood. Discard contaminated shoes. Do not breathe vapor.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances. Corrosives area.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 617-33-4: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: clear, colorless
Odor: none reported
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 77 deg C @ 12.00mm Hg
Freezing/Melting Point: Not available.
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature: Not available.
Solubility in water: Not available.
Specific Gravity/Density: 1.9020g/cm3
Molecular Formula: C4H6Br2O2
Molecular Weight: 245.90
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, strong oxidants.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 617-33-4 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Acetic acid, dibromo-, ethyl ester - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
No information available.
IMO
No information available.
RID/ADR
No information available.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 617-33-4: No information available.
Canada
CAS# 617-33-4 is listed on Canada's NDSL List.
CAS# 617-33-4 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 617-33-4 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 二溴乙酸 dibromoacetic acid 631-64-1 C2H2Br2O2 217.845
反应信息
-
作为反应物:描述:参考文献:名称:Schaeffer, Chemische Berichte, 1871, vol. 4, p. 366摘要:DOI:
-
作为产物:描述:参考文献:名称:Magnani; McElvain, Journal of the American Chemical Society, 1938, vol. 60, p. 2212摘要:DOI:
文献信息
-
Fe(0)-Mediated Synthesis of Tri- and Tetra-Substituted Olefins from Carbonyls: An Environmentally Friendly Alternative to Cr(II)作者:J. R. Falck、Romain Bejot、Deb K. Barma、Anish Bandyopadhyay、Suju Joseph、Charles MioskowskiDOI:10.1021/jo061445u日期:2006.10.1carbonyls by activated polyhalides. In many instances, Fe(0) was equivalent or superior to Cr(II). Notably, Fe(0), but not Cr(II), proved compatible with a wide range of functionality, inter alia, unprotected phenol, aryl nitro, carboxylic acid, and alkyl nitrile. A surprising reversal of stereoselectivity for aldehydes versus ketones was observed using both metals. The resultant α-halo-α,β-unsaturated or α
-
Vicarious Nucleophilic Substitution of Nitrobenzenes作者:Gerath A. DeBoos、David J. MilnerDOI:10.1080/00397919408020772日期:1994.4Abstract Vicarious nucleophilic substitution (VNS) by dichloroacetate and other reagents has been applied in good yield to various ortho-substituted nitrobenzenes including 2-nitrotoluene and 2-nitro ethylbenzene. For alkyl nitrobenzenes, ease of VNS increased para < ortho < meta and methyl < ethyl < iso-propyl. Formation of chlorine-free products upon VNS by methyl dichloroacetate suggests the involvement
-
The Addition Reaction of Samarium Enolates and 2-Haloenolates Derived from Esters, and Amides to Imines. Totally Stereoselective Synthesis of Enantiopure 3,4-Diamino Esters or Amides作者:Joséâ M. Concellón、Humberto RodrÃguez-Solla、Carmen Simal、Vicente del Amo、Santiago GarcÃa-Granda、M. Rosario DÃazDOI:10.1002/adsc.200900534日期:2009.11addition reaction of samarium enolates and 2-haloenolates derived from esters and amides to imines takes place in an efficient manner. A novel protocol to perform the addition reaction of samarium enolates derived from esters or amides to chiral 2-aminoimines, with total stereoselectivity and without racemization, is also reported. The use of samarium enolates in place of other classic metallic enolates
-
Molecular water oxidation catalysis by zwitterionic carboxylate bridge-functionalized bis-NHC iridium complexes作者:Raquel Puerta-Oteo、M. Victoria Jiménez、Jesús J. Pérez-TorrenteDOI:10.1039/c8cy02306a日期:——transformed into the same active molecular species resulting from the degradation of the hydrocarbon ligands which is partially supported by the similarity of the oxygen evolution profiles at moderate oxidant/catalyst ratios for both catalyst precursors and the same chemical oxidant. In addition, DLS studies provide evidence for the participation of homogeneous iridium molecular species as intermediates likely两性离子水溶性[Cp * Ir III Cl (MeIm)2 CHCOO}]和[Ir I(cod)(MeIm)2 CHCOO}]配合物,具有羧酸酯桥官能化的双-N-杂环卡宾配体,可有效催化水使用硝酸铈铈(IV)或高碘酸钠作为牺牲氧化剂进行氧化。TOF 50值高达1000 h -1时,产量极高使用CAN作为电子受体以[CAN] / [Ir]的比率高于700已经实现了这一目标。通过紫外可见光谱对反应机理的研究似乎证明了两种催化剂前体均因降解而转化为相同的活性分子。对于催化剂前体和相同的化学氧化剂,在适中的氧化剂/催化剂比下,由氧释放曲线的相似性部分支持了烃配体的一部分。此外,DLS研究提供了均质铱分子物质作为中间体的证据,这些中间体很可能被羧酸盐官能化的双-NHC配体稳定。
-
一种唑来膦酸中间体化合物
表征谱图
-
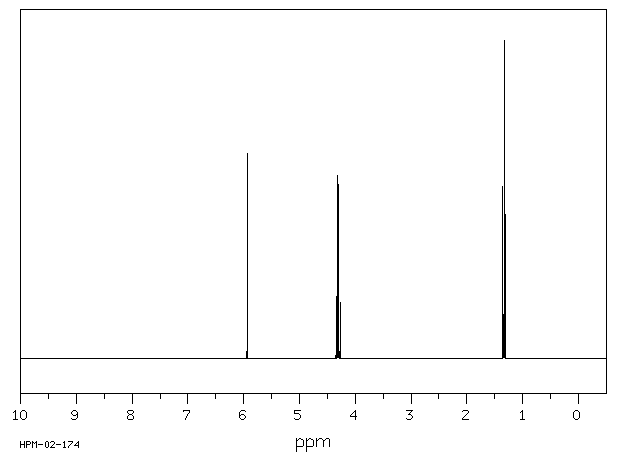
氢谱1HNMR
-
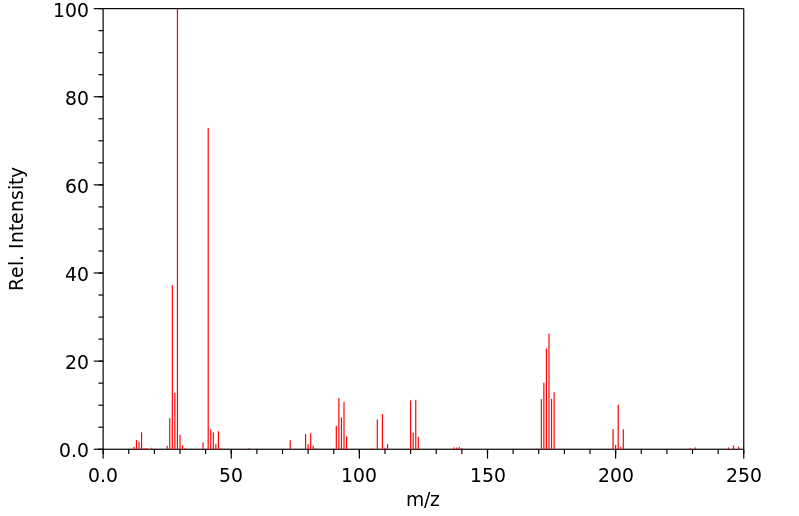
质谱MS
-
碳谱13CNMR
-
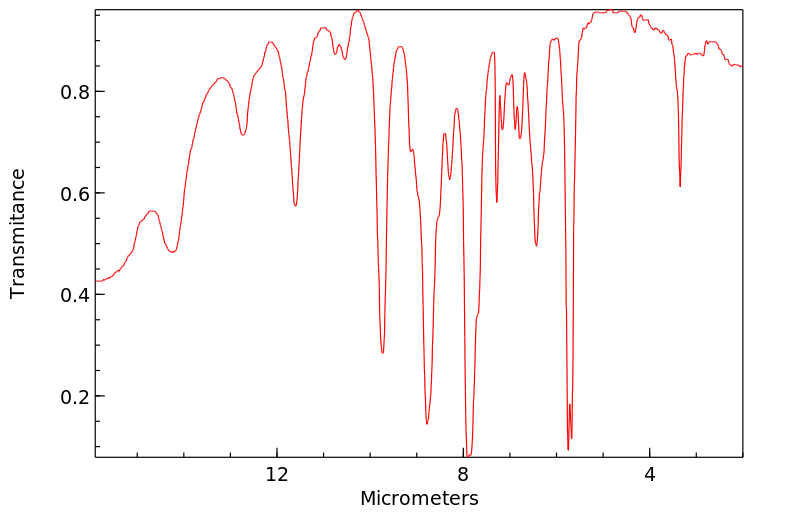
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸