花生酸酐 | 55726-22-2
中文名称
花生酸酐
中文别名
二十烷酸酐
英文名称
arachidic anhydride
英文别名
eicosanoic acid-anhydride;Eicosansaeure-anhydrid;icosanoyl icosanoate
CAS
55726-22-2
化学式
C40H78O3
mdl
MFCD00056235
分子量
607.058
InChiKey
AUONNNVJUCSETH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:77 °C
-
沸点:563.3±18.0 °C(Predicted)
-
密度:0.880±0.06 g/cm3(Predicted)
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解,未有已知危险反应。
计算性质
-
辛醇/水分配系数(LogP):18.9
-
重原子数:43
-
可旋转键数:38
-
环数:0.0
-
sp3杂化的碳原子比例:0.95
-
拓扑面积:43.4
-
氢给体数:0
-
氢受体数:3
安全信息
-
海关编码:2915900090
-
储存条件:密封,在-20°C下保存
SDS
花生酸酐
模块 1. 化学品
产品名称: Arachidic Anhydride
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 花生酸酐
百分比: >98.0%(T)
CAS编码: 55726-22-2
俗名: Eicosanoic Anhydride
花生酸酐
模块 3. 成分/组成信息
分子式: C40H78O3
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
存放于惰性气体环境中。
防湿。
远离不相容的材料比如氧化剂存放。
潮敏
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
花生酸酐
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色-浅灰色
气味: 无资料
pH: 无数据资料
熔点: 77°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
花生酸酐
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: Arachidic Anhydride
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 花生酸酐
百分比: >98.0%(T)
CAS编码: 55726-22-2
俗名: Eicosanoic Anhydride
花生酸酐
模块 3. 成分/组成信息
分子式: C40H78O3
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
存放于惰性气体环境中。
防湿。
远离不相容的材料比如氧化剂存放。
潮敏
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
花生酸酐
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色-浅灰色
气味: 无资料
pH: 无数据资料
熔点: 77°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
花生酸酐
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 硬脂酸酐 stearic anhydride 638-08-4 C36H70O3 550.95
反应信息
-
作为反应物:参考文献:名称:新型喜树碱衍生物,10-羟基喜树碱的长链脂肪酸酯(作为有效的前药候选物)的合成及其通过羧酸酯酶的体外代谢转化。摘要:制备了五种带有长链脂肪酸酯的(20S)-10-羟基喜树碱衍生物,用于开发新型的前药型药物。使用来自大鼠,猪和人的肝微粒体的三种纯化的羧酸酯酶同工酶进行的体外实验表明,与CPT-11相比,这些衍生物可通过酶有效地代谢。DOI:10.1016/s0960-894x(98)00039-0
-
作为产物:参考文献:名称:Bleyberg; Ulrich, Chemische Berichte, 1931, vol. 64, p. 2511摘要:DOI:
文献信息
-
Studies on antitumor agents. VI. Syntheses and antitumor activities of acyl derivatives of 2'-deoxy-5-trifluoromethyluridine.作者:JUN-ICHI YAMASHITA、SETSUO TAKEDA、HIROSHI MATSUMOTO、TADAFUMI TERADA、NORIO UNEMI、MITSUGI YASUMOTODOI:10.1248/cpb.35.2090日期:——Various O-acyl and N-acyl derivatives of 2'-deoxy-5-trifluoromethyluridine (F3Thd) were synthesized; namely 5'-O-acyl, 3', 5'-di-O-acyl, N3-acyl, 3', 5'-di-O-acetyl-N3-acyl, 3', 5'-di-O-carbamoyl and 3', 5'-di-O-ethoxycarbonyl compounds. 5'-O-Acyl derivatives of 2'-deoxy-5-trifluoromethylcytidine were also synthesized. The antitumor activities of these compounds against sarcoma 180 were examined by oral administration to mice. Among the 5'-and 3', 5'-diester compounds with aliphatic acids, the 5'-O-hexanoyl compound showed the highest activity. Full protection of the sugar moiety with aroyl or carbamoyl groups considerably decreased the activities, and those of the 3', 5'-di-O-m-fluorobenzoyl and 3, 5'-di-O-butylcarbamoyl compounds were the smallest. N3-Benzoyl compounds were slightly more effective than F3Thd but none of them showed higher activity than the effective O-acyl compounds. In the case of 5'-O-acylates of 2'-deoxy-5-trifluoromethylcytidine, the 5'-O-benzoyl compound showed the highest activity.合成了2'-脱氧-5-三氟甲基尿苷(F3Thd)的各种O-酰基和N-酰基衍生物,即5'-O-酰基、3', 5'-二-O-酰基、N3-酰基、3',5'-二-O-乙酰基-N3-酰基、3', 5'-二-O-氨甲酰基和3',5'-二-O-乙氧羰基化合物。还合成了2'-脱氧-5-三氟甲基胞苷的5'-O-酰基衍生物。通过给小鼠口服给药,考察了这些化合物对肉瘤180的抗癌活性。在具有脂肪族酸的5'-和3'5'-二酯化合物中,5'-O-己酰基化合物显示出最高的活性。用芳酰基或氨甲酰基保护糖部分的完全保护大大降低了活性,而3',5'-二-O-间氟苯甲酰基和3',5'-二-O-丁基氨甲酰基化合物的活性最小。N3-苯甲酰基化合物的效力略高于F3Thd,但它们中没有任何一种显示出比有效的O-酰基化合物更高的活性。在2'-脱氧-5-三氟甲基胞苷的5'-O-酰基化合物中,5'-O-苯甲酰基化合物显示出最高的活性。
-
Potentiation of the Antitumor Effect of 5-Fluoro-2′-deoxyuridine Esters in Combination with Acyclothymidine Esters on L1210 in Mice via Oral Administration作者:Takeo Kawaguchi、Masahiko Saito、Mineo SaneyoshiDOI:10.1002/jps.2600771108日期:1988.11acids in order to synchronize their behavior after simultaneous oral administration. The antitumor activity of orally administered FUdR esters was potentiated by the simultaneous oral administration of the acyclothymidine esters, but not by acyclothymidine.
-
Antitumor composition comprising pyrimidine type nucleic acid derivative申请人:Teijin Limited公开号:US04916121A1公开(公告)日:1990-04-10An antitumor agent comprising (i) at least one pyrimidine type nucleic acid derivative having the general formula (I): ##STR1## wherein X represents a fluorine atom or a trifluoromethyl group, R.sup.1 and R.sup.2 may be the same or different and represent a hydrogen atom or an acyl group, R.sup.3 represents a hydrogen atom or a hydroxyl group, and (ii) at least one 1-[(2-hydroxyethoxy)methyl]uracil derivative having the general formula (II): ##STR2## wherein Y represents a hydrogen atom, a halogen atom, a methyl group, a benzyl group, a benzyloxybenzyl group, a vinyl group, a nitro group, or an amino group and R.sup.4 represents a hydrogen atom or an acyl group.
-
Cytidine nucleotide derivatives申请人:Asahi Kasei Kogyo Kabushiki Kaisha公开号:US04086417A1公开(公告)日:1978-04-25A nucleotide derivative represented by the general formula: ##STR1## wherein R represents an acyl group having 14 - 22 carbon atoms and having an aliphatic acyl moiety; X represents a hydrogen atom, a halogen atom, an alkyl group having 1 - 4 carbon atoms, or a trifluoromethyl group; and at least one, but not all, of Y.sub.1, Y.sub.2, and Y.sub.3 represents a phosphate, a pyrophosphate, a triphosphate, or a salt thereof, the balance of Y.sub.1, Y.sub.2 and Y.sub.3 representing a hydroxyl group; or in case of ribosyl, Y.sub.1 and Y.sub.3, or Y.sub.2 and Y.sub.3 represent, at the same time, the aforesaid phosphate, pyrophosphate, triphosphate or salt thereof and the remaining group of Y.sub.1, Y.sub.2 or Y.sub.3 represents a hydroxyl group; or in case of arabinosyl, Y.sub.1 and Y.sub.2, or Y.sub.2 and Y.sub.3 represent, at the same time, the aforesaid phosphate, pyrophosphate, triphosphate or salt therof and the remaining group of Y.sub.1, Y.sub.2 or Y.sub.3 represents a hydroxyl group. The nontoxic salts of these phosphates are useful as an antileukemialagent for mice which is soluble in water and has high stability.一种核苷酸衍生物,其通式表示为:##STR1## 其中R代表具有14-22个碳原子和具有脂肪族酰基的酰基基团;X代表氢原子、卤素原子、具有1-4个碳原子的烷基基团或三氟甲基基团;至少一个但不是全部的Y.sub.1、Y.sub.2和Y.sub.3代表磷酸盐、焦磷酸盐、三磷酸盐或其盐,Y.sub.1、Y.sub.2和Y.sub.3的平衡代表羟基;在核糖基的情况下,Y.sub.1和Y.sub.3,或者Y.sub.2和Y.sub.3同时代表上述磷酸盐、焦磷酸盐、三磷酸盐或其盐,而Y.sub.1、Y.sub.2或Y.sub.3的其余基团代表羟基;在阿拉伯糖基的情况下,Y.sub.1和Y.sub.2,或者Y.sub.2和Y.sub.3同时代表上述磷酸盐、焦磷酸盐、三磷酸盐或其盐,而Y.sub.1、Y.sub.2或Y.sub.3的其余基团代表羟基。这些磷酸盐的无毒盐在水中溶解并具有高稳定性,可用作小鼠的抗白血病剂。
-
Antitumor agent申请人:TEIJIN LIMITED公开号:EP0202056A1公开(公告)日:1986-11-20An antitumor agent comprising (i) at least one pyrimidine type nucleic acid derivative having the general formula (I): wherein X represents a fluorine atom or a trifluoromethyl group, R' and R2 may be the same or different and represent a hydrogen atom or an acyl group, R3 represents a hydrogen atom or a hydroxyl group, and (ii) at least one 1-[(2-hydroxyethoxy)methyl]uraci derivative having the general formula (II): wherein Y represents a hydrogen atom, a halogen atom, a methyl group, a benzyl group, a benzyloxybenzyl group, a vinyl group, a nitro group, or an amino group and R4 represents a hydrogen atom or an acyl group.
表征谱图
-
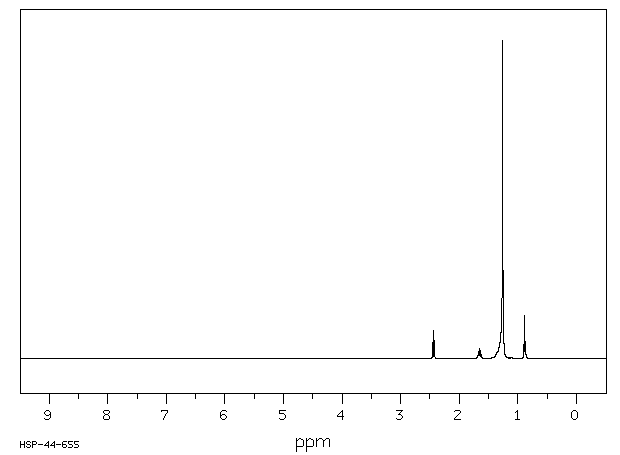
氢谱1HNMR
-
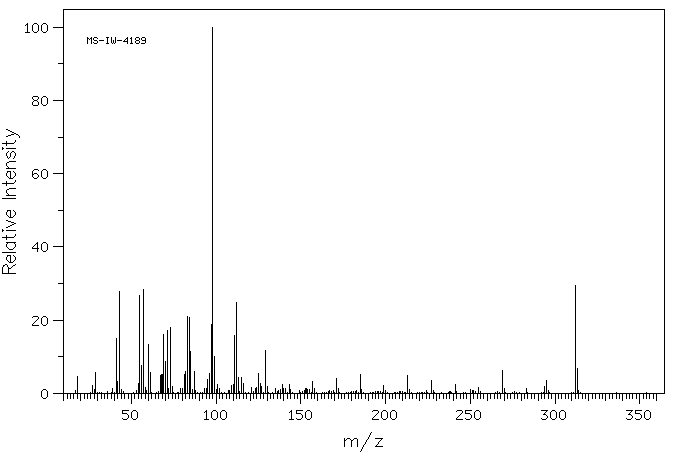
质谱MS
-
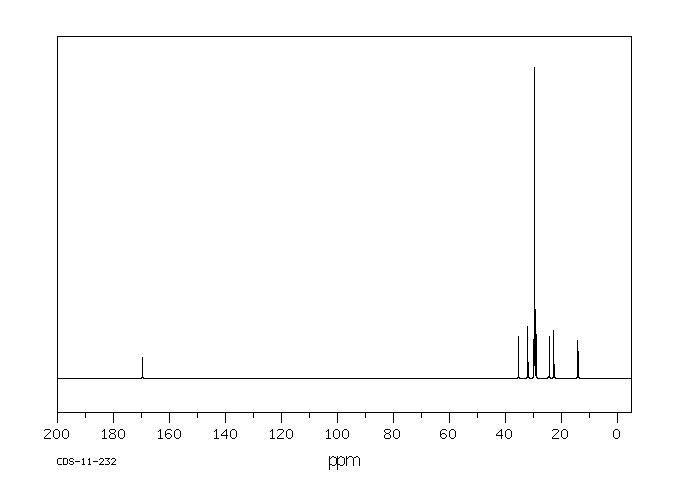
碳谱13CNMR
-
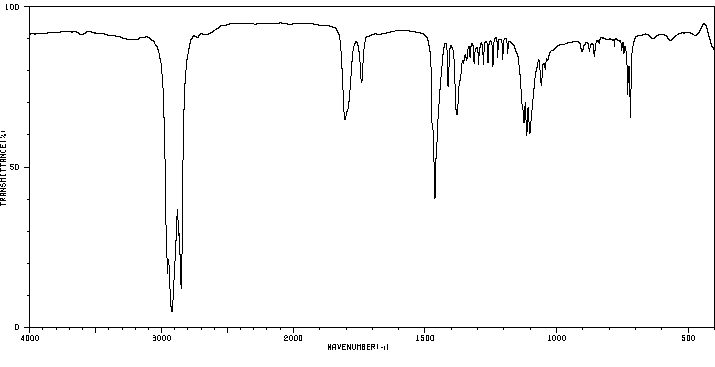
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸