双(2,4,6-三甲基苯基)二硒化物 | 71518-92-8
中文名称
双(2,4,6-三甲基苯基)二硒化物
中文别名
——
英文名称
dimesityl diselenide
英文别名
1,2-dimesityldiselane;1,3,5-trimethyl-2-[(2,4,6-trimethylphenyl)diselanyl]benzene
CAS
71518-92-8
化学式
C18H22Se2
mdl
——
分子量
396.293
InChiKey
CCLBDRJGGUNRSE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:115 °C(Solv: ethanol (64-17-5))
-
沸点:458.3±55.0 °C(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.81
-
重原子数:20
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.33
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
反应信息
-
作为反应物:描述:双(2,4,6-三甲基苯基)二硒化物 在 sodium tetrahydroborate 作用下, 以 N,N-二甲基甲酰胺 为溶剂, 反应 0.5h, 生成 2,4,6-Trimethylbenzeneselenol参考文献:名称:炔烃的立体选择性还原:4-有机硒基喹啉的合成摘要:本研究描述了 2-氨基芳炔基酮与有机硒醇盐反应形成 ( Z )-乙烯基硒化物,其通过分子内缩合生成 4-有机硒基喹啉。使用优化的反应条件,用各种芳基炔基酮和二有机基二硒化物研究了这种环化的普遍性。对反应机理的研究导致了乙烯基硒化物的分离和鉴定,它是该环化的关键中间体。为了扩大结构多样性并证明制备的 4-有机硒基喹啉的适用性,我们研究了它们作为底物在使用n裂解碳 - 硒键中的应用-丁基锂,然后通过亲电试剂和 Suzuki 和 Sonogashira 交叉偶联反应捕获锂中间体。DOI:10.1021/acs.joc.2c01255
-
作为产物:描述:参考文献:名称:探索 L-碲半胱氨酸、Te 保护的碲半胱氨酸缀合物和二有机二硒化物对过氧化氢的反应性:合成和分子结构分析摘要:L-碲半胱氨酸 [Te 2 {CH 2 CH(NH 3 + )COO - } 2 ] ( 4 ) 与 H 2 O 2在 HBr 存在下的氧化反应导致形成环状的两性离子有机碲酸盐( IV )种,即[Te - {CH 2 CH(NH 3 + )COO}(Br) 3 ] ( 5 )。Te-保护的碲半胱氨酸衍生物 [RTe{CH 2 CH(NH 2 )COOH}] [R = C]的 H 2 O 2氧化6 H 5 ( 6 ) 和 4-MeC 6 H 4 ( 7 )] 在 HCl 存在下形成氯碲烷物质 [(C 6 H 5 )Te{CH 2 CH(NH 3 + )COOH}(Cl ) 2 ]Cl ( 8 ) 和 [(4-MeC 6 H 5 )Te{CH 2 CH(NH 3 + )COO}(Cl)]Cl ( 9 )。二有机二硒化物的氧化反应 R 2 Se 2 [R = 4-MeC 6 H 4 ( 10),DOI:10.1039/d2nj00997h
-
作为试剂:描述:参考文献:名称:Oxidation of alcohols with tert-butyl hydroperoxide and diaryl diselenide摘要:DOI:10.1021/jo00344a017
文献信息
-
Organoselenium compounds from purines: Synthesis of 6-arylselanylpurines with antioxidant and anticholinesterase activities and memory improvement effect作者:Luis Fernando B. Duarte、Renata L. Oliveira、Karline C. Rodrigues、Guilherme T. Voss、Benhur Godoi、Ricardo F. Schumacher、Gelson Perin、Ethel A. Wilhelm、Cristiane Luchese、Diego AlvesDOI:10.1016/j.bmc.2017.11.019日期:2017.12We describe here a simple method for the synthesis of 6-arylselanylpurines with antioxidant and anticholinesterase activities, and memory improvement effect. This class of compounds was synthesized in good yields by a reaction of 6-chloropurine with diaryl diselenides using NaBH4 as reducing agent and PEG-400 as solvent. Furthermore, the synthesized compounds were evaluated for their in vitro antioxidant
-
Transition metal-free coupling reactions of benzylic trimethylammonium salts with di(hetero)aryl disulfides and diselenides作者:Fuhai Li、Dan Wang、Hongyi Chen、Ze He、Lihong Zhou、Qingle ZengDOI:10.1039/d0cc05633b日期:——A new protocol was developed to synthesize (enantioenriched) thioethers and selenoethers from (chiral) benzylic trimethylammonium salts and di(hetero)aryl disulfides or diselenides. These syntheses were promoted by the presence of weak base and did not require the use of any transition metal, and resulted in the target products with good to excellent yields (72–94%). Using quaternary ammonium salts
-
A greener protocol for the synthesis of phosphorochalcogenoates: Antioxidant and free radical scavenging activities作者:Daniela H. Mailahn、Lucas E.B. Iarocz、Patrick C. Nobre、Gelson Perin、Airton Sinott、Ana Paula Pesarico、Paloma T. Birmann、Lucielli Savegnago、Márcio S. SilvaDOI:10.1016/j.ejmech.2020.113052日期:2021.3which this stability study was also important to select some products for pharmacological screening. The phosphorochalcogenoates were screened in vitro and ex vivo tests for the antioxidant potential and free radical scavenging activity, as well as to investigation toxicity in mice through of the plasma levels of markers of renal and hepatic damage. The pharmacological screening of phosphorochalcogenoates在这一贡献中,已开发出一种无金属和碱的方案,用于通过在50°C下使用DMSO作为溶剂来合成磷碳氢酸盐(Se和Te)。由二有机基二卤代膦酸酯和H-膦酸酯制备了多种磷代碳氢酸酯,导致了Chal-P(O)键的形成,反应速度快,收率好至极佳。通过1D和2D NMR,IR,CGMS和HRMS分析获得了产品的完整结构说明,并进行了磷藻酸酯的稳定性评估以有效地描述这种简单可行的方法。典型77硒 1个H}(δ硒= 866.0 pPM)设为125碲 1个H}(δTE = 422.0 PPM)和31 p 1个H}(δ P = -1.0,-13.0 -15.0和PPM)NMR化学位移均必须确认的副产物,其中该稳定性研究也很重要选择一些产品的药理学筛选。在体外和离体中筛选了磷酸胆碱酯进行抗氧化剂潜力和自由基清除活性测试,并通过血浆肾脏和肝损伤标志物的水平调查小鼠的毒性。药代磷酸酯的药理学筛选表明,该化合物
-
Ultrasound-promoted regioselective synthesis of chalcogeno-indolizines by a stepwise 1,3-dipolar cycloaddition作者:Marcelo M. Vieira、Bianca T. Dalberto、Felipe L. Coelho、Paulo H. SchneiderDOI:10.1016/j.ultsonch.2020.105228日期:2020.11moderate to excellent yields from pyridinium salts and chalcogeno-alkynes. The reaction can be carried out under thermal conditions or by sonochemical processes in short reaction times. The stepwise cycloaddition reaction forming chalcogeno-indolizines is regioselective and extends to a broad range of functional groups. Furthermore, novel chalcogeno-alkynes are reported and the first derivatives of
-
Trapping of Payne rearrangement intermediates with arylselenide anions作者:Michael E. Jung、Daniel L. SunDOI:10.1016/j.tetlet.2014.11.103日期:2015.6The intermediate epoxy alcohols prepared via a Payne rearrangement can be trapped with arylselenide anions, giving mixtures of ring-opened products. The 1-arylseleno-2,3-diols are generally favored over the 3-arylseleno-1,2-diols in this process although the reaction of trisubstituted epoxyalcohols, for example, 17, differs from those of disubstituted epoxyalcohols, for example, 21.
表征谱图
-
氢谱1HNMR
-
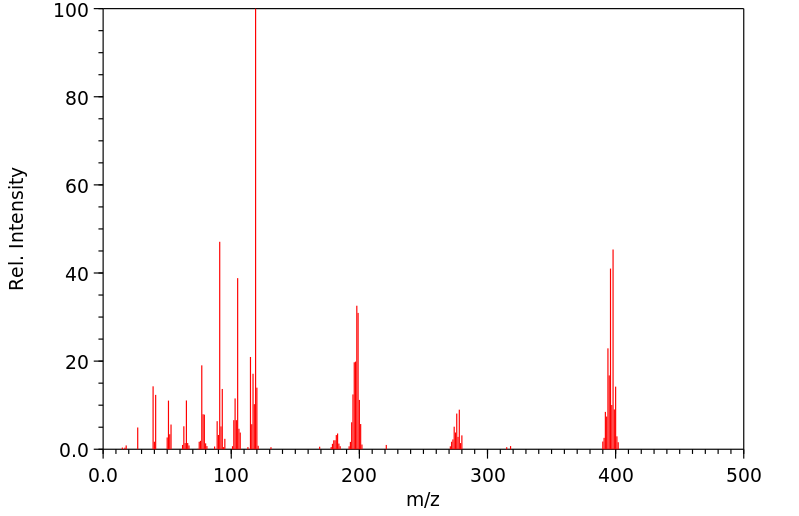
质谱MS
-
碳谱13CNMR
-
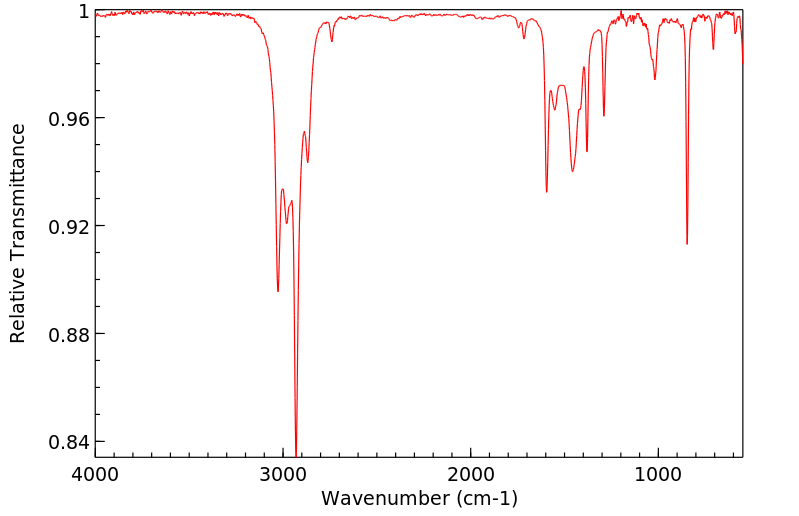
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫