3,5-二异丙基邻羟基苯甲酸 | 2215-21-6
中文名称
3,5-二异丙基邻羟基苯甲酸
中文别名
3,5-二异丙基水杨酸
英文名称
3,5-diisopropylsalicylic acid
英文别名
2-hydroxy-3,5-diisopropyl benzoic acid;2-hydroxy-3,5-diisopropylbenzoic acid;3,5-diisopropyl-2-hydroxybenzoic acid;2-hydroxy-3,5-diisopropylbenzoic;3,5-di-iso-propyl salicylic acid;2-hydroxy-3,5-di(propan-2-yl)benzoic acid
CAS
2215-21-6
化学式
C13H18O3
mdl
MFCD00008884
分子量
222.284
InChiKey
XUFUYOGWFZSHGE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:113-115 °C (lit.)
-
沸点:303.46°C (rough estimate)
-
密度:1.1205 (rough estimate)
-
溶解度:易溶于有机溶剂。
-
稳定性/保质期:
避免让氧化物直接接触。
计算性质
-
辛醇/水分配系数(LogP):5.5
-
重原子数:16
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.461
-
拓扑面积:57.5
-
氢给体数:2
-
氢受体数:3
安全信息
-
TSCA:Yes
-
危险品标志:Xi
-
安全说明:S22,S26,S37/39
-
危险类别码:R36/37
-
WGK Germany:3
-
海关编码:2918290000
-
危险品运输编号:NONH for all modes of transport
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H319,H335
-
储存条件:请将容器密封后,存放在干燥、阴凉处。
SDS
| Name: | 3 5-Diisopropylsalicylic Acid 98% Material Safety Data Sheet |
| Synonym: | Benzoic Acid, 2-Hydroxy-3,5-Bis(1-Methylethyl)- |
| CAS: | 2215-21-6 |
Synonym:Benzoic Acid, 2-Hydroxy-3,5-Bis(1-Methylethyl)-
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 2215-21-6 | Benzoic Acid, 2-Hydroxy-3,5-Bis(1-Meth | 98 | 218-677-8 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 2215-21-6: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: beige to gray
Odor: none reported
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 117.00 - 119.00 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C13H18O3
Molecular Weight: 222.28
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Strong bases, strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 2215-21-6 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Benzoic Acid, 2-Hydroxy-3,5-Bis(1-Methylethyl)- - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 2215-21-6: No information available.
Canada
CAS# 2215-21-6 is listed on Canada's NDSL List.
CAS# 2215-21-6 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 2215-21-6 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-hydroxy-3,5-diisopropyl-benzoic acid methyl ester 53434-12-1 C14H20O3 236.311 1-(2-羟基-3,5-二异丙基苯基)乙酮 2-acetyl-4,6-di-iso-propylphenol 35158-23-7 C14H20O2 220.312 —— 2-acetoxy-3,5-diisopropylbenzoic acid 74152-39-9 C15H20O4 264.321 —— 4-carbomethoxyphenyl 3,5-diisopropylsalicylate 49669-40-1 C21H24O5 356.419 —— 2-Benzoyloxy-3,5-di(propan-2-yl)benzoic acid 1415567-00-8 C20H22O4 326.392 —— 2-hydroxy-3,5-di(propan-2-yl)benzamide 33978-55-1 C13H19NO2 221.299 2,4-二异丙基苯酚 2,4-diisopropylphenol 2934-05-6 C12H18O 178.274 —— 3-(2-hydroxy-3,5-diisopropyl-benzoylamino)butyric acid 870287-14-2 C17H25NO4 307.39
反应信息
-
作为反应物:描述:3,5-二异丙基邻羟基苯甲酸 在 草酰氯 、 N,N-二甲基甲酰胺 、 氨 作用下, 以 二氯甲烷 为溶剂, 反应 1.0h, 以40%的产率得到2-hydroxy-3,5-di(propan-2-yl)benzamide参考文献:名称:[EN] SUBSTITUTED FUROCHROMENE COMPOUNDS OF ANTIINFLAMMATORY ACTION
[FR] COMPOSES DE FUROCHROMENE SUBSTITUES A ACTION ANTI-INFLAMMATOIRE摘要:本发明涉及公式(I)的新化合物,包括所有立体异构体和互变异构体,以及其药用可接受盐和溶剂合物,用于预防和治疗人类的哮喘和其他炎症性疾病和/或情况的过程和反应中间体,以及它们的使用。公开号:WO2005010006A1 -
作为产物:参考文献:名称:Hydrazide salts摘要:具有通式##SPC1##的化合物,其中R.sup.1,R.sup.2,R.sup.3和R.sup.4可以相同也可以不同,代表氢,具有1至20个碳原子的直链或支链烷基基团,具有7至20个碳原子的芳基烷基基团,可以被一个或多个具有1至4个碳原子的烷基基团取代,或者可以是以下类型之一:##SPC2## 其中R.sup.5和R.sup.6分别是氢原子或甲基基团,每个R代表R.sup.1,R.sup.2,R.sup.3和R.sup.4的其他基团,具有2至20个碳原子的烯基基团;具有5至12个碳原子的环烷基基团,可以被一个或多个具有1至4个碳原子的烷基基团取代;具有6至10个碳原子的芳基基团;具有7至12个碳原子的烷基芳基基团;具有2或3个碳原子的烷基基团和环己烯基环可能包含一个甲基基团作为取代基的环己基烷基基团;具有2或3个碳原子的烯基环己基基团,其中烯基基团具有2或3个碳原子,环己基环也可能包含一个甲基基团作为取代基;或者是一个-OR.sup.7基团,其中R.sup.7代表具有1至20个碳原子的烷基基团,具有5至12个碳原子的环烷基基团,可以选择一个或多个具有1至4个碳原子的烷基基团取代,具有6至10个碳原子的芳基基团,或具有3至20个碳原子的烯基基团;或者任意两个相邻的基团R.sup.1,R.sup.2,R.sup.3和R.sup.4可以连接在一起形成一个芳基环,该芳基环可以被一个或多个具有1至20个碳原子的烷基基团取代;X代表阴离子;n是X的价,该化合物对于从水溶液中提取金属是有用的。公开号:US03932505A1
文献信息
-
[EN] NICOTINE SALTS, CO-CRYSTALS, AND SALT CO-CRYSTAL COMPLEXES<br/>[FR] SELS, CO-CRISTAUX, ET COMPLEXES DE CO-CRISTAUX DE SELS DE NICOTINE申请人:REYNOLDS TOBACCO CO R公开号:WO2015183801A1公开(公告)日:2015-12-03The invention provides certain nicotine salts, co-crystals, and salt co-crystals and provides novel polymorphic forms of certain nicotine salts. In particular, nicotine salts with mucic acid, 3,5-dihydroxybenzoic acid, and 2,3-dihydroxybenzoic acid, and crystalline polymorphic forms of nicotine 4-acetamidobenzoate, nicotine gentisate, and nicotine 1-hydroxy-2-naphthoate are described. The invention further provides methods of preparation and characterization of such nicotine salts, co-crystals, and salt co-crystals and polymorphic forms thereof. In addition, tobacco products, including smoking articles, smokeless tobacco products, and electronic smoking articles comprising nicotine salts, co-crystals, and/or salt co-crystals are also provided.
-
[EN] CHROMENE DERIVATIVES AND USE THEREOF AS HIF HYDROXYLASE ACTIVITY INHIBITORS<br/>[FR] DÉRIVÉS DE CHROMÈNE ET LEUR UTILISATION EN TANT QU'INHIBITEURS DE L'ACTIVITÉ DE L'HYDROXYLASE HIF申请人:FIBROGEN INC公开号:WO2009100250A1公开(公告)日:2009-08-13The present invention relates to novel compounds of formula (I), methods, and compositions capable of decreasing HIF hydroxylase activity, thereby increasing the stability and/or activity of hypoxia inducible factor (HIF).本发明涉及公式(I)的新化合物,以及能够降低HIF羟化酶活性的方法和组合物,从而增加缺氧诱导因子(HIF)的稳定性和/或活性。
-
Benzimidazolidinone derivatives as muscarinic agents申请人:——公开号:US20030100545A1公开(公告)日:2003-05-29Benzimidazolidinone derivative compounds, which increase acetylcholine signaling or effect in the brain, and highly selective muscarinic agonists, particularly for the M 1 and/or M 4 receptor subtypes, pharmaceutical compositions comprising the same, as well as methods of treating psychosis using these compounds are disclosed.
-
Hexameric Organotincarboxylates with Cyclic and Drum Structures作者:Ganesan Prabusankar、Ramaswamy MurugavelDOI:10.1021/om049584u日期:2004.11.1(1H, 13C, and 119Sn) spectroscopy. The molecular structure of 1 determined by single-crystal X-ray diffraction studies reveals a rare cyclic hexameric structure. The tin atoms are pentacoordinated with distorted trigonal-bipyramidal geometry. Complex 2 is also a hexameric cluster, but existing in a more common drum-like structure where the tin centers are hexacoordinated.3,5-二异丙基水杨酸(DIPSA)与[ n苯中的Bu 2 Sn(O)]进行除水处理,生成锡氧烷[ nBu 2 Sn(3,5- iPr 2 C 6 H 2(O)(COO))] 6(1),而DIPSA与[ nBuSn(O)(OH)]导致[ nBuSn(O)(3,5- iPr 2 C 6 H 2(OH)(COO))] 6(2)。这些新产品已通过元素分析和EI-MS,IR和NMR(1 H,13 C和119 Sn)光谱进行了表征。通过单晶X射线衍射研究确定的分子结构1揭示了一种罕见的环状六聚体结构。锡原子以扭曲的三角双锥体几何形状五配位。配合物2也是六聚体簇,但以锡中心为六配位的更常见的鼓状结构存在。
-
Metal complexes of alkylating agents. IV. Complexes of melphalan and chlorambucil作者:Melvin D. Joesten、Ethel L. Krasney、Jamie B. NeidertDOI:10.1016/s0020-1693(00)80557-5日期:1989.5Abstract Complexes of melphalan (mel) and chlorambucil (cam) that have been synthesized include Cu(mel)2(H2O)2, Cu(sal-mel)(sal) where sal-mel is the Schiff base formed from salicylic acid and melphalan, Pt(cyc)2(mel)2 where cyc is cyclohexylamine, (H3C)2Au(mel), and Cu2(cam)4.
表征谱图
-
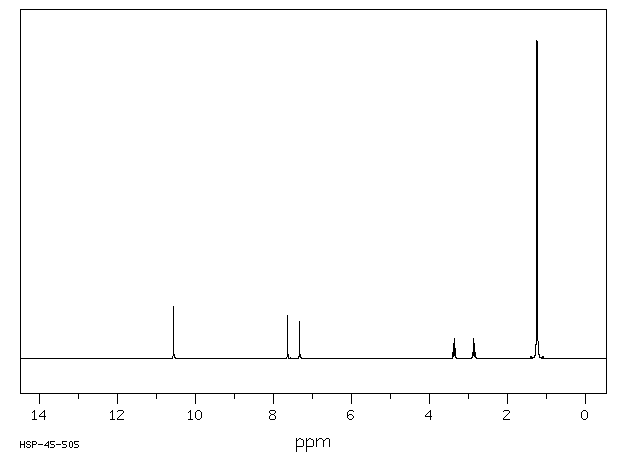
氢谱1HNMR
-
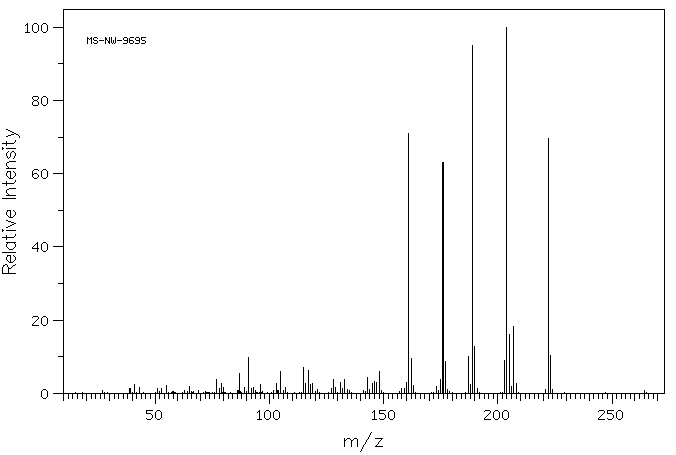
质谱MS
-
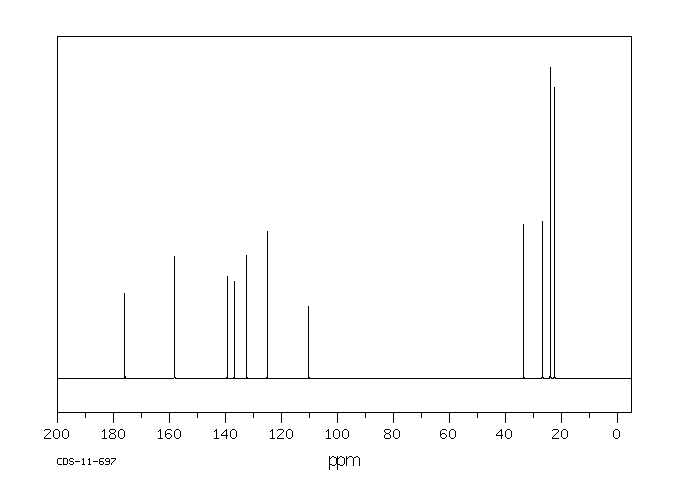
碳谱13CNMR
-
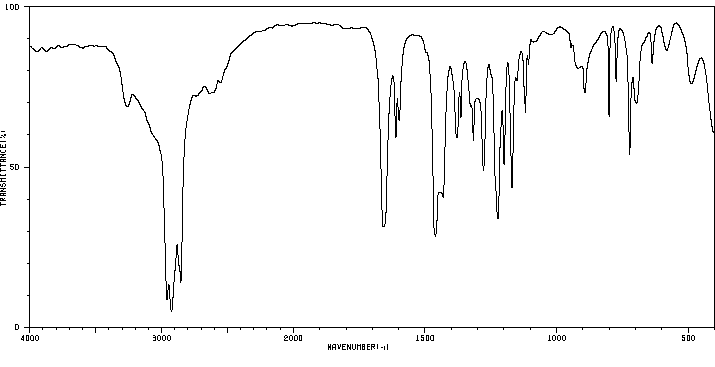
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫