2-氯吡啶-4-硼酸频哪醇酯 | 458532-84-8
中文名称
2-氯吡啶-4-硼酸频哪醇酯
中文别名
2-氯-吡啶-4-硼酸频那酯;2-氯-吡啶-4-硼酸频哪醇酯
英文名称
2-chloro-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridine
英文别名
2-chloropyridine-4-boronic acid pinacol ester
CAS
458532-84-8
化学式
C11H15BClNO2
mdl
MFCD04039870
分子量
239.51
InChiKey
UUEQDBHKMOFLDP-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:64.7-64.8°C
-
沸点:338.2±27.0 °C(Predicted)
-
密度:1.14±0.1 g/cm3(Predicted)
-
稳定性/保质期:
按规定使用和贮存不会分解,避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):3.48
-
重原子数:16
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.545
-
拓扑面积:31.4
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
安全说明:S26,S45
-
危险类别码:R25,R36
-
海关编码:2934999090
-
危险品运输编号:UN 2811
-
危险标志:GHS06
-
危险性描述:H301,H319
-
危险性防范说明:P301 + P310,P305 + P351 + P338
-
储存条件:请将药品存放在密闭、阴凉、干燥的地方。
SDS
SECTION 1: Identification of the substance/mixture and of the company/undertaking
Product identifiers
Product name : 2-Chloro-4-(4,4,5,5-Tetramethyl-1,3,2-Dioxaborolan-
2-Yl)Pyridine
REACH No. : A registration number is not available for this substance as the substance
or its uses are exempted from registration, the annual tonnage does not
require a registration or the registration is envisaged for a later
registration deadline.
Relevant identified uses of the substance or mixture and uses advised against
Identified uses : Laboratory chemicals, Manufacture of substances
SECTION 2: Hazards identification
Classification of the substance or mixture
Classification according to Regulation (EC) No 1272/2008
Acute toxicity, Oral (Category 3), H301
Eye irritation (Category 2), H319
For the full text of the H-Statements mentioned in this Section, see Section 16.
Classification according to EU Directives 67/548/EEC or 1999/45/EC
T Toxic R25, R36
For the full text of the R-phrases mentioned in this Section, see Section 16.
Label elements
Labelling according Regulation (EC) No 1272/2008
Pictogram
Signal word Danger
Hazard statement(s)
H301 Toxic if swallowed.
H319 Causes serious eye irritation.
Precautionary statement(s)
P301 + P310 IF SWALLOWED: Immediately call a POISON CENTER or doctor/
physician.
P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove
contact lenses, if present and easy to do. Continue rinsing.
Supplemental Hazard none
Statements
Other hazards
This substance/mixture contains no components considered to be either persistent, bioaccumulative and
toxic (PBT), or very persistent and very bioaccumulative (vPvB) at levels of 0.1% or higher.
SECTION 3: Composition/information on ingredients
Substances
Molecular weight : 239,51 g/mol
Hazardous ingredients according to Regulation (EC) No 1272/2008
Component Classification Concentration
2-Chloro-4-(4,4,5,5-Tetramethyl-1,3,2-Dioxaborolan-2-Yl)Pyridine
Acute Tox. 3; Eye Irrit. 2; <= 100 %
H301, H319
Hazardous ingredients according to Directive 1999/45/EC
Component Classification Concentration
2-Chloro-4-(4,4,5,5-Tetramethyl-1,3,2-Dioxaborolan-2-Yl)Pyridine
T, R25 - R36 <= 100 %
For the full text of the H-Statements and R-Phrases mentioned in this Section, see Section 16
SECTION 4: First aid measures
Description of first aid measures
General advice
Consult a physician. Show this safety data sheet to the doctor in attendance.
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician.
In case of skin contact
Wash off with soap and plenty of water. Take victim immediately to hospital. Consult a physician.
In case of eye contact
Rinse thoroughly with plenty of water for at least 15 minutes and consult a physician.
If swallowed
Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician.
Most important symptoms and effects, both acute and delayed
The most important known symptoms and effects are described in the labelling (see section 2.2) and/or in
section 11
Indication of any immediate medical attention and special treatment needed
No data available
SECTION 5: Firefighting measures
Extinguishing media
Suitable extinguishing media
Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.
Special hazards arising from the substance or mixture
Nature of decomposition products not known.
Advice for firefighters
Wear self-contained breathing apparatus for firefighting if necessary.
Further information
No data available
SECTION 6: Accidental release measures
Personal precautions, protective equipment and emergency procedures
Wear respiratory protection. Avoid dust formation. Avoid breathing vapours, mist or gas. Ensure
adequate ventilation. Evacuate personnel to safe areas. Avoid breathing dust.
For personal protection see section 8.
Environmental precautions
Prevent further leakage or spillage if safe to do so. Do not let product enter drains.
Methods and materials for containment and cleaning up
Pick up and arrange disposal without creating dust. Sweep up and shovel. Keep in suitable, closed
containers for disposal.
Reference to other sections
For disposal see section 13.
SECTION 7: Handling and storage
Precautions for safe handling
Avoid contact with skin and eyes. Avoid formation of dust and aerosols.
Provide appropriate exhaust ventilation at places where dust is formed.
For precautions see section 2.2.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place.
Storage class (TRGS 510): Non-combustible, acute toxic Cat.3 / toxic hazardous materials or hazardous
materials causing chronic effects
Specific end use(s)
Apart from the uses mentioned in section 1.2 no other specific uses are stipulated
SECTION 8: Exposure controls/personal protection
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
Avoid contact with skin, eyes and clothing. Wash hands before breaks and immediately after handling
the product.
Personal protective equipment
Eye/face protection
Face shield and safety glasses Use equipment for eye protection tested and approved under
appropriate government standards such as NIOSH (US) or EN 166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and
the standard EN 374 derived from it.
Body Protection
Complete suit protecting against chemicals, The type of protective equipment must be selected
according to the concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
Where risk assessment shows air-purifying respirators are appropriate use a full-face particle
respirator type N99 (US) or type P2 (EN 143) respirator cartridges as a backup to engineering
controls. If the respirator is the sole means of protection, use a full-face supplied air respirator. Use
respirators and components tested and approved under appropriate government standards such
as NIOSH (US) or CEN (EU).
Control of environmental exposure
Prevent further leakage or spillage if safe to do so. Do not let product enter drains.
SECTION 9: Physical and chemical properties
Information on basic physical and chemical properties
a) Appearance Form: solid
b) Odour No data available
c) Odour Threshold No data available
d) pH No data available
e) Melting point/freezing No data available
point
f) Initial boiling point and No data available
boiling range
g) Flash point No data available
h) Evaporation rate No data available
i) Flammability (solid, gas) No data available
j) Upper/lower No data available
flammability or
explosive limits
k) Vapour pressure No data available
l) Vapour density No data available
m) Relative density No data available
n) Water solubility No data available
o) Partition coefficient: n- No data available
octanol/water
p) Auto-ignition No data available
temperature
q) Decomposition No data available
temperature
r) Viscosity No data available
s) Explosive properties No data available
t) Oxidizing properties No data available
Other safety information
No data available
SECTION 10: Stability and reactivity
Reactivity
No data available
Chemical stability
Stable under recommended storage conditions.
Possibility of hazardous reactions
No data available
Conditions to avoid
No data available
Incompatible materials
Strong oxidizing agents
Hazardous decomposition products
In the event of fire: see section 5
SECTION 11: Toxicological information
Information on toxicological effects
Acute toxicity
No data available
Skin corrosion/irritation
No data available
Serious eye damage/eye irritation
No data available
Respiratory or skin sensitisation
No data available
Germ cell mutagenicity
No data available
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
No data available
Specific target organ toxicity - single exposure
No data available
Specific target organ toxicity - repeated exposure
No data available
Aspiration hazard
No data available
Additional Information
RTECS: Not available
To the best of our knowledge, the chemical, physical, and toxicological properties have not been
thoroughly investigated.
SECTION 12: Ecological information
Toxicity
No data available
Persistence and degradability
No data available
Bioaccumulative potential
No data available
Mobility in soil
No data available
Results of PBT and vPvB assessment
This substance/mixture contains no components considered to be either persistent, bioaccumulative and
toxic (PBT), or very persistent and very bioaccumulative (vPvB) at levels of 0.1% or higher.
Other adverse effects
No data available
SECTION 13: Disposal considerations
Waste treatment methods
Product
Offer surplus and non-recyclable solutions to a licensed disposal company. Dissolve or mix the material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber.
Contaminated packaging
Dispose of as unused product.
SECTION 14: Transport information
UN number
ADR/RID: 2811 IMDG: 2811 IATA: 2811
UN proper shipping name
ADR/RID: TOXIC SOLID, ORGANIC, N.O.S. (2-Chloro-4-(4,4,5,5-Tetramethyl-1,3,2-Dioxaborolan-2-
Yl)Pyridine)
IMDG: TOXIC SOLID, ORGANIC, N.O.S. (2-Chloro-4-(4,4,5,5-Tetramethyl-1,3,2-Dioxaborolan-2-
Yl)Pyridine)
IATA: Toxic solid, organic, n.o.s. (2-Chloro-4-(4,4,5,5-Tetramethyl-1,3,2-Dioxaborolan-2-Yl)Pyridine)
Transport hazard class(es)
ADR/RID: 6.1 IMDG: 6.1 IATA: 6.1
Packaging group
ADR/RID: III IMDG: III IATA: III
Environmental hazards
ADR/RID: no IMDG Marine pollutant: no IATA: no
Special precautions for user
No data available
SECTION 15: Regulatory information
This safety datasheet complies with the requirements of Regulation (EC) No. 1907/2006.
Safety, health and environmental regulations/legislation specific for the substance or mixture
No data available
Chemical Safety Assessment
For this product a chemical safety assessment was not carried out
SECTION 16: Other information
Full text of H-Statements referred to under sections 2 and 3.
Acute Tox. Acute toxicity
Eye Irrit. Eye irritation
H301 Toxic if swallowed.
H319 Causes serious eye irritation.
Full text of R-phrases referred to under sections 2 and 3
T Toxic
R25 Toxic if swallowed.
R36 Irritating to eyes.
Further information
Copyright 2014 Co. LLC. License granted to make unlimited paper copies for internal use
only.
The above information is believed to be correct but does not purport to be all inclusive and shall be
used only as a guide. The information in this document is based on the present state of our knowledge
and is applicable to the product with regard to appropriate safety precautions. It does not represent any
guarantee of the properties of the product. Corporation and its Affiliates shall not be held
liable for any damage resulting from handling or from contact with the above product. See
and/or the reverse side of invoice or packing slip for additional terms and conditions of sale.
反应信息
-
作为反应物:描述:2-氯吡啶-4-硼酸频哪醇酯 在 copper(II) bis(trifluoromethanesulfonate) 、 4-(dimethylamino)pyridinium trifluoromethanesulfonate 、 氢氟酸 作用下, 以 N,N-二甲基乙酰胺 为溶剂, 反应 0.33h, 生成参考文献:名称:一种不添加碱的铜介导放射性氟化的共沸免干燥方法摘要:铜介导的放射性氟化为芳烃和杂芳烃的 18 F 标记提供了一种快速且通用的方法。然而,已知这种方法对碱敏感,这一直是制备型放射合成的障碍。在本报告中,我们提供了一种无需共沸干燥或添加碱的铜介导放射性氟化的方法。用 4-二甲氨基吡啶鎓三氟甲磺酸盐 (DMAP·OTf) 在无水 N,N-二甲基甲酰胺 (DMF) 中的溶液定量洗脱 PS-HCO3 Sep-Pak 上捕获的 [ 18 F] 氟化物。洗脱的溶液直接用于铜介导的放射性氟化。测试了 12 种硼酸酯底物,基于 HPLC 分析,氟化产物的放射化学产率为 27% 至 83%。该方法成功应用于[ 18 F]氟马西尼的放射合成,一种众所周知的正电子发射断层扫描 (PET) 示踪剂,用于对中枢苯二氮卓受体进行成像,放射化学产率为 47%。这种高效的协议显着增强了强大的铜介导的放射性氟化方法。DOI:10.1002/jlcr.3705
-
作为产物:参考文献:名称:Selective inhibitors of rock protein kinase and uses thereof摘要:本文描述了作为ROCK抑制剂有用的化合物。这些化合物及其药学上可接受的组合物对于治疗或减轻各种疾病的严重程度是有用的,包括心血管、炎症、神经系统或增殖性疾病或疾病。公开号:US20070270386A1
文献信息
-
[EN] PYRIDYL UREAS AS MINERALOCORTICOID RECEPTOR ANTAGONISTS<br/>[FR] URÉES DE PYRIDYL COMME ANTAGONISTES DE RÉCEPTEUR MINÉRALOCORTICOÏDE申请人:BOEHRINGER INGELHEIM INT公开号:WO2012064631A1公开(公告)日:2012-05-18Mineralocorticoid receptor antagonists, of which formula (1) is exemplary.矿物皮质激素受体拮抗剂,其中公式(1)是示例。
-
[EN] BIARYL KINASE INHIBITORS<br/>[FR] INHIBITEURS DE KINASES À BASE DE BIARYLE申请人:BRISTOL MYERS SQUIBB CO公开号:WO2017059085A1公开(公告)日:2017-04-06The present disclosure is directed to biaryl compounds of formula (I) which can inhibit AAKl (adaptor associated kinase 1), compositions comprising such compounds and their use for treating e.g. pain, Alzheimer's disease, Parkinson's disease and schizophrenia.
-
Discovery of 4-(((4-(5-chloro-2-(((1s,4s)-4-((2-methoxyethyl)amino)cyclohexyl)amino)pyridin-4-yl)thiazol-2-yl)amino)methyl)tetrahydro-2H-pyran-4-carbonitrile (JSH-150) as a novel highly selective and potent CDK9 kinase inhibitor作者:Beilei Wang、Jiaxin Wu、Yun Wu、Cheng Chen、Fengming Zou、Aoli Wang、Hong Wu、Zhenquan Hu、Zongru Jiang、Qingwang Liu、Wei Wang、Yicong Zhang、Feiyang Liu、Ming Zhao、Jie Hu、Tao Huang、Juan Ge、Li Wang、Tao Ren、Yuxin Wang、Jing Liu、Qingsong LiuDOI:10.1016/j.ejmech.2018.09.025日期:2018.10Through a structure-guided rational drug design approach, we have discovered a highly selective inhibitor compound 40 (JSH-150), which exhibited an IC50 of 1 nM against CDK9 kinase in the biochemical assay and achieved around 300–10000-fold selectivity over other CDK kinase family members. In addition, it also displayed high selectivity over other 468 kinases/mutants (KINOMEscan S score(1) = 0.01)通过结构指导的合理药物设计方法,我们发现了一种高度选择性的抑制剂化合物40(JSH-150),在生化分析中对CDK9激酶的IC 50为1 nM,相对于CDK9激酶具有约300-10000倍的选择性。其他CDK激酶家族成员。此外,它还显示出对其他468种激酶/突变体的高选择性(KINOMEscan S得分(1)= 0.01)。化合物40显示出对黑素瘤,神经母细胞瘤,肝癌,结肠癌,肺癌以及白血病细胞系的有效抗增殖作用。它可以剂量依赖性地抑制RNA Pol II的磷酸化,抑制MCL-1和c-Myc的表达,阻滞细胞周期并诱导白血病细胞凋亡。在MV4-11细胞接种的异种移植小鼠模型中,10 mg / kg剂量的40剂量几乎可以完全抑制肿瘤的进展。高选择性和良好的体内PK / PD特性表明40是研究CDK9介导的生理学和病理学的良好药理工具,也是白血病和其他癌症的潜在候选药物。
-
Synthesis of Biaryls through a One-Pot Tandem Borylation/Suzuki-Miyaura Cross-Coupling Reaction Catalyzed by a Palladacycle作者:Lianhui Wang、Xiuling Cui、Jingya Li、Yusheng Wu、Zhiwu Zhu、Yangjie WuDOI:10.1002/ejoc.201101409日期:2012.1The tricyclohexylphosphane adduct of cyclopalladated ferrocenylimine I exhibited high catalytic activity in the one-pot borylation/Suzuki-Miyaura coupling (BSC) reaction with low catalyst loading (2 mol-%). Various biaryls were obtained in good to excellent yields for 37 examples. This process was applied to aryl and heteroaryl halides (Br and Cl) containing a variety of functional groups and did not
-
Iridium-catalyzed C–H borylation of pyridines作者:Scott A. Sadler、Hazmi Tajuddin、Ibraheem A. I. Mkhalid、Andrei S. Batsanov、David Albesa-Jove、Man Sing Cheung、Aoife C. Maxwell、Lena Shukla、Bryan Roberts、David C. Blakemore、Zhenyang Lin、Todd B. Marder、Patrick G. SteelDOI:10.1039/c4ob01565g日期:——The iridium-catalysed CâH borylation is a valuable and attractive method for the preparation of aryl and heteroaryl boronates. However, application of this methodology for the preparation of pyridyl and related azinyl boronates can be challenged by low reactivity and propensity for rapid protodeborylation, particularly for a boronate ester ortho to the azinyl nitrogen. Competition experiments have revealed that the low reactivity is due to inhibition of the active catalyst through coordination of the azinyl nitrogen lone pair at the vacant site on the iridium. This effect can be overcome through the incorporation of a substituent at C-2. Moreover, when this is sufficiently electron-withdrawing protodeborylation is sufficiently slowed to permit isolation and purification of the C-6 boronate ester. Following functionalization, reduction of the directing C-2 substituent provides the product arising from formal ortho borylation of an unhindered pyridine ring.
表征谱图
-
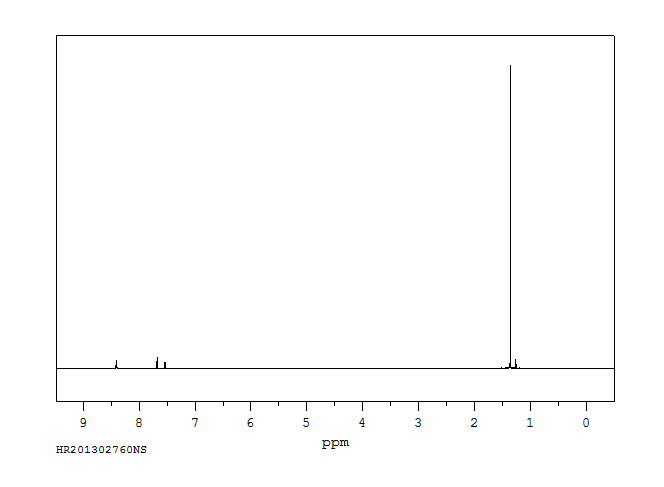
氢谱1HNMR
-
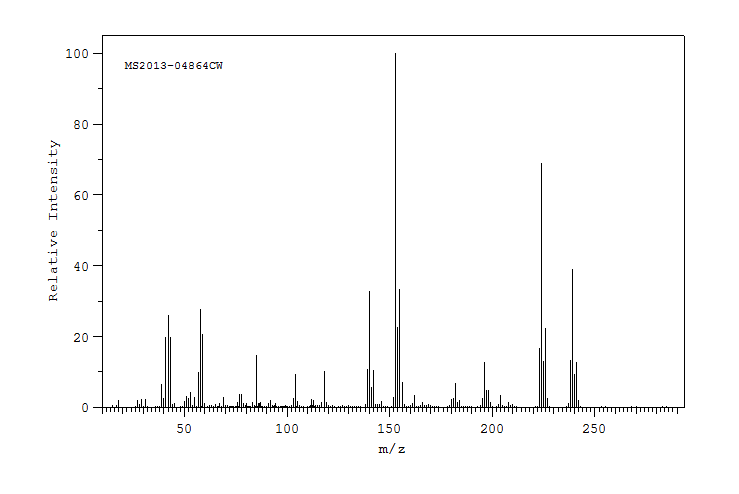
质谱MS
-
碳谱13CNMR
-
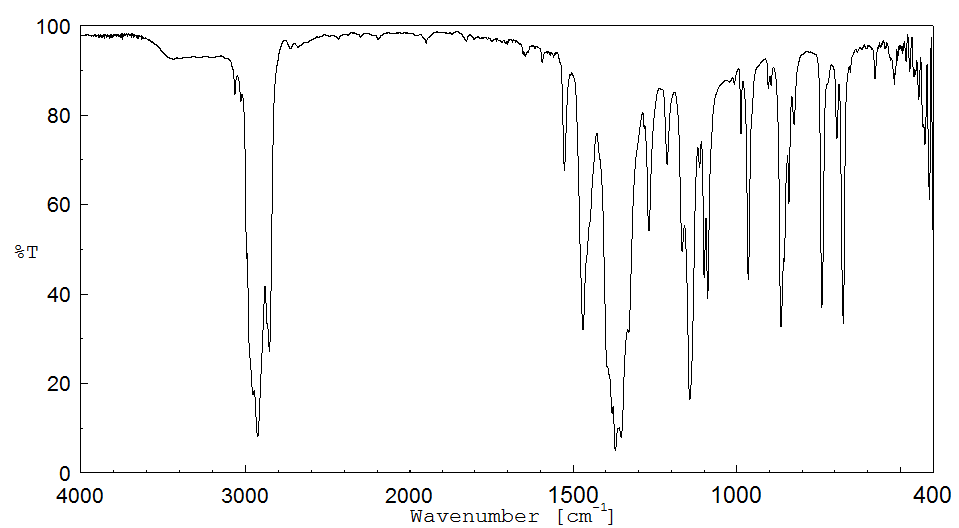
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-氨氯地平-d4
(R,S)-可替宁N-氧化物-甲基-d3
(R)-(+)-2,2'',6,6''-四甲氧基-4,4''-双(二苯基膦基)-3,3''-联吡啶(1,5-环辛二烯)铑(I)四氟硼酸盐
(R)-N'-亚硝基尼古丁
(R)-DRF053二盐酸盐
(5E)-5-[(2,5-二甲基-1-吡啶-3-基-吡咯-3-基)亚甲基]-2-亚磺酰基-1,3-噻唑烷-4-酮
(5-溴-3-吡啶基)[4-(1-吡咯烷基)-1-哌啶基]甲酮
(5-氨基-6-氰基-7-甲基[1,2]噻唑并[4,5-b]吡啶-3-甲酰胺)
(2S,2'S)-(-)-[N,N'-双(2-吡啶基甲基]-2,2'-联吡咯烷双(乙腈)铁(II)六氟锑酸盐
(2S)-2-[[[9-丙-2-基-6-[(4-吡啶-2-基苯基)甲基氨基]嘌呤-2-基]氨基]丁-1-醇
(2R,2''R)-(+)-[N,N''-双(2-吡啶基甲基)]-2,2''-联吡咯烷四盐酸盐
(1'R,2'S)-尼古丁1,1'-Di-N-氧化物
黄色素-37
麦斯明-D4
麦司明
麝香吡啶
鲁非罗尼
鲁卡他胺
高氯酸N-甲基甲基吡啶正离子
高氯酸,吡啶
高奎宁酸
马来酸溴苯那敏
马来酸氯苯那敏-D6
马来酸左氨氯地平
顺式-双(异硫氰基)(2,2'-联吡啶基-4,4'-二羧基)(4,4'-二-壬基-2'-联吡啶基)钌(II)
顺式-二氯二(4-氯吡啶)铂
顺式-二(2,2'-联吡啶)二氯铬氯化物
顺式-1-(4-甲氧基苄基)-3-羟基-5-(3-吡啶)-2-吡咯烷酮
顺-双(2,2-二吡啶)二氯化钌(II) 水合物
顺-双(2,2'-二吡啶基)二氯化钌(II)二水合物
顺-二氯二(吡啶)铂(II)
顺-二(2,2'-联吡啶)二氯化钌(II)二水合物
韦德伊斯试剂
非那吡啶
非洛地平杂质C
非洛地平
非戈替尼
非布索坦杂质66
非尼拉朵
非尼拉敏
雷索替丁
阿雷地平
阿瑞洛莫
阿扎那韦中间体
阿培利司N-6
阿伐曲波帕杂质40
间硝苯地平
间-硝苯地平
镉,二碘四(4-甲基吡啶)-
锌,二溴二[4-吡啶羧硫代酸(2-吡啶基亚甲基)酰肼]-