2-甲氧基肉桂醛 | 60125-24-8
中文名称
2-甲氧基肉桂醛
中文别名
3-(2-甲氧基苯基)-2-丙烯醛
英文名称
(E)-3-(2-methoxyphenyl)propenal
英文别名
(E)-3-(2-methoxyphenyl)acrylaldehyde;2-Methoxycinnamaldehyde;o-methoxycinnamaldehyde;trans-2-methoxycinnamaldehyde;(E)-2-methoxycinnamaldehyde;2-methoxy-trans-cinnamaldehyde;trans-o-methoxycinnamaldehyde;2-methoxy-(E)-cinnamaldehyde;(E)-o-methoxycinnamaldehyde;o-methoxycinnamic aldehyde;O-Methoxycinnamaldehyde, (E)-;(E)-3-(2-methoxyphenyl)prop-2-enal
CAS
60125-24-8
化学式
C10H10O2
mdl
MFCD00007001
分子量
162.188
InChiKey
KKVZAVRSVHUSPL-GQCTYLIASA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:44-48 °C(lit.)
-
沸点:160-161 °C12 mm Hg(lit.)
-
密度:1.0281 (rough estimate)
-
闪点:>230 °F
-
LogP:2.370 (est)
-
物理描述:O-methoxycinnamaldehyde appears as yellow crystals. (NTP, 1992)
-
溶解度:less than 0.1 mg/mL at 64° F (NTP, 1992)
-
保留指数:1512;1504
-
稳定性/保质期:
遵照规定使用和储存,则不会发生分解。
计算性质
-
辛醇/水分配系数(LogP):1.7
-
重原子数:12
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.1
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险品标志:Xi
-
危险类别码:R36/37/38
-
WGK Germany:2
-
RTECS号:GD6590000
-
安全说明:S26,S37/39
-
危险性防范说明:P261,P264,P271,P280,P302+P352,P304+P340,P305+P351+P338,P312,P313,P337+P313,P362,P403+P233,P405,P501
-
危险性描述:H315,H319,H335
-
储存条件:保持贮藏器密封,并将其放入一个紧密封装的容器中。应储存在阴凉、干燥的地方。
SDS
制备方法与用途
制备方法:GB2760-1997规定为允许使用的食品用香料。
用途简介:目前暂无具体用途信息。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2'-甲氧基肉桂醛 2-methoxycinnamaldehyde 1504-74-1 C10H10O2 162.188 顺-2-甲氧基肉桂酸 (Z)-o-methoxycinnamic acid 14737-91-8 C10H10O3 178.188 反式-2-甲氧基肉桂酸 2-methoxycinnamic acid 1011-54-7 C10H10O3 178.188 —— (E)-1-(buta-1,3-dien-1-yl)-2-methoxybenzene 79554-84-0 C11H12O 160.216 —— (E)-3-(2-methoxyphenyl)prop-2-en-1-ol —— C10H12O2 164.204 —— methyl 2-methoxycinnamate 98288-15-4 C11H12O3 192.214 —— (Z)-ethyl 3-(2-methoxyphenyl)acrylate 289473-81-0 C12H14O3 206.241 2-戊酮,4-氨基-3-甲基-,(3R,4S)-rel- ethyl 3-(2-methoxyphenyl)prop-2-enoate 24393-54-2 C12H14O3 206.241 1-[(1E)-3,3-二甲氧基-1-丙烯-1-基]-2-甲氧基苯 1-(3,3-Dimethoxyprop-1-en-1-yl)-2-methoxybenzene 433936-29-9 C12H16O3 208.257 —— 1-[(E)-3,3-dimethoxyprop-1-enyl]-2-methoxybenzene 1171019-85-4 C12H16O3 208.257 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 (E)-2-甲氧基肉桂酰胺 (E)-3-(2-methoxyphenyl)acrylamide 144393-52-2 C10H11NO2 177.203 反式-2-甲氧基肉桂酸 2-methoxycinnamic acid 1011-54-7 C10H10O3 178.188 (3E)-4-(2-甲氧基苯基)-3-丁烯-2-酮 (E)-4-(2-methoxyphenyl)but-3-en-2-one 60438-50-8 C11H12O2 176.215 —— (E)-1-(buta-1,3-dien-1-yl)-2-methoxybenzene 79554-84-0 C11H12O 160.216 —— (E)-3-(2-Methoxyphenyl)-2-propenylamine 158223-63-3 C10H13NO 163.219 —— (E)-3-(2-methoxyphenyl)prop-2-en-1-ol —— C10H12O2 164.204 —— (E)-1-(3-bromoprop-1-en-1-yl)-2-methoxybenzene 194342-93-3 C10H11BrO 227.101 (E)-3-(2-甲氧基苯基)丙烯腈 (E)-3-(2-methoxyphenyl)acrylonitrile 57103-26-1 C10H9NO 159.188 —— methyl 2-methoxycinnamate 98288-15-4 C11H12O3 192.214 —— (2E,4E)-5-(2-methoxyphenyl)penta-2,4-dienal 115754-60-4 C12H12O2 188.226 —— (E)-4-(2-methoxyphenyl)but-3-enoic acid 127404-71-1 C11H12O3 192.214 —— (E)-1-(2-methoxyphenyl)-3-phenylpropene —— C16H16O 224.302 —— (E)-3-(2-methoxyphenyl)allyl methyl carbonate 512789-12-7 C12H14O4 222.241 —— (+/-)-(3E)-1-chloro-4-(2-methoxy-phenyl)-but-3-en-2-ol 399513-48-5 C11H13ClO2 212.676 —— ethyl (2E,4E)-5-(2-methoxyphenyl)penta-2,4-dienoate 1019654-18-2 C14H16O3 232.279 —— (1E,3R)-1-(2-methoxyphenyl)hexa-1,5-dien-3-ol 1260607-50-8 C13H16O2 204.269 —— (1E,3S)-1-(2-methoxyphenyl)hexa-1,5-dien-3-ol 1260609-35-5 C13H16O2 204.269 —— (2E,5R,6E)-5-hydroxy-7-(2-methoxyphenyl)-2-methylhepta-2,6-dienenitrile 1380225-25-1 C15H17NO2 243.305 —— (E)-N-(tert-butyl)-4-(2-methoxyphenyl)-2-oxobut-3-enamide 1320256-58-3 C15H19NO3 261.321 —— 2-methoxy-cinnamylideneacetophenone 139435-32-8 C18H16O2 264.324 - 1
- 2
反应信息
-
作为反应物:参考文献:名称:Lewis碱催化的α,β-不饱和醛向饱和羧酸,酯和酰胺的转化摘要:在催化量的路易斯碱(如TTMPP和DBU)存在下,α,β-不饱和醛(如(E)-肉桂醛和2-或4-甲氧基肉桂醛)与三甲基甲硅烷基氰化物反应,水解后得到相应的饱和羧酸。DOI:10.1016/s0040-4039(02)01133-4
-
作为产物:描述:(E)-1-(buta-1,3-dien-1-yl)-2-methoxybenzene 在 ferric sulfate heptahydrate 、 氧气 作用下, 以 水 、 乙腈 为溶剂, 反应 18.0h, 以91%的产率得到2-甲氧基肉桂醛参考文献:名称:铁(III)/ O2介导的1-芳基丁二烯向肉桂醛的区域选择性氧化裂解。摘要:已经开发了由硫酸铁(III)/ O 2介导的1-芳基丁二烯对肉桂醛的简单,有效和环境友好的区域选择性氧化裂解。该反应提供了良好的产率和优异的区域选择性,并显示出良好的官能团耐受性(31个实施例)。该方法很重要,因为很少有报道报道可对共轭二烯进行如此出色的氧化裂解的底物范围有限。DOI:10.1021/acs.orglett.9b03562
文献信息
-
MnO<sub>2</sub> as a terminal oxidant in Wacker oxidation of homoallyl alcohols and terminal olefins作者:Rodney A. Fernandes、Gujjula V. Ramakrishna、Venkati BethiDOI:10.1039/d0ob01344g日期:——PdCl2/CrO3/HCl produced α,β-unsaturated ketones from homoallyl alcohols, the present method provided orthogonally the β-hydroxy-methyl ketones. No overoxidation or elimination of benzylic and/or β-hydroxy groups was observed. The method could be extended to the oxidation of simple terminal olefins as well, to methyl ketones, displaying its versatility. An application to the regioselective synthesis of gingerol is
-
Tandem Oxidation-Dehydrogenation of (Hetero)Arylated Primary Alcohols via Perruthenate Catalysis作者:Christian J. Bettencourt、Sharon Chow、Peter W. Moore、Christopher D. G. Read、Yanxiao Jiao、Jan Peter Bakker、Sheng Zhao、Paul V. Bernhardt、Craig M. WilliamsDOI:10.1071/ch21137日期:——Tandem oxidative-dehydrogenation of primary alcohols to give α,β-unsaturated aldehydes in one pot are rare transformations in organic synthesis, with only two methods currently available. Reported herein is a novel method using the bench-stable salt methyltriphenylphosphonium perruthenate (MTP3), and a new co-oxidant NEMO·PF6 (NEMO = N-ethyl-N-hydroxymorpholinium) which provides unsaturated aldehydes伯醇的串联氧化脱氢在一锅中得到 α,β-不饱和醛是有机合成中罕见的转化,目前只有两种方法可用。本文报道了一种使用长期稳定的过钌酸甲基三苯基鏻盐 (MTP3) 和新的助氧化剂 NEMO·PF 6 (NEMO = N-乙基-N-羟基吗啉) 的新方法,该方法以低到中等的收率提供不饱和醛。使用N-氧化物助氧化剂 NMO (NMO = N-甲基吗啉N-氧化物)/NEMO对(杂)芳基化伯醇进行 Ley-Griffith 氧化,通过添加N-氧化物盐 NEMO·PF 6得到扩展将中间体饱和醛转化为其不饱和对应物。重点介绍了该方法的发现、方法开发、反应范围和相关挑战。通过合成与辅助结合蛋白 B 相关的多烯支架,证明了天然产物合成后期脱氢的概念价值。
-
Ruthenium-Catalyzed Rearrangement of Aldoximes to Primary Amides in Water作者:Rocío García-Álvarez、Alba E. Díaz-Álvarez、Javier Borge、Pascale Crochet、Victorio CadiernoDOI:10.1021/om3006917日期:2012.9.10The rearrangement of aldoximes to primary amides has been studied using the readily available arene-ruthenium(II) complex [RuCl2(η6-C6Me6)P(NMe2)3}] (5 mol %) as catalyst. Reactions proceeded cleanly in pure water at 100 °C without the assistance of any cocatalyst, affording the desired amides in high yields (70–90%) after short reaction times (1–7 h). The process was operative with both aromatic
-
Design and synthesis of a library of tertiary amides: Evaluation as mimetics of the melanocortins’ active core作者:Felikss Mutulis、Jana Kreicberga、Sviatlana Yahorava、Ilze Mutule、Larisa Borisova-Jan、Aleh Yahorau、Ruta Muceniece、Sandra Azena、Santa Veiksina、Ramona PetrovskaDOI:10.1016/j.bmc.2007.06.003日期:2007.9.1acceptable results. According to this approach an efficient formation of Schiff bases was achieved in the presence of TiCl(4). Substances were isolated by reversed phase chromatography; in some cases isomers were additionally separated by chiral chromatography on Chirobiotic T. When tested on human recombinant melanocortin receptors all the tertiary amides showed some binding affinities; for the highest affinity在固相上制备了201种叔酰胺。将二胺偶联到活化的羧化王聚合物上,并使所得的聚合物取代的苄氧羰基保护的二胺与原甲酸三甲酯中的醛或酮反应,得到树脂连接的席夫碱。然后将偶联的树脂通过在4%的乙酸/原甲酸三甲酯中的氰基硼氢化钠还原为仲胺,然后在PyBroP和二异丙基乙胺的存在下用羧酸酰化。在清除剂(主要是1,2-乙二硫醇)存在下,通过三氟乙酸从树脂上裂解叔酰胺。当制备吲哚衍生物时,与连接体片段发生平行烷基化,得到2-(4-羟基苄基)-吲哚的衍生物作为副产物。在上述方法未给出可接受的结果的情况下,标题化合物的溶液合成或液相/固相混合制备被证明是有利的。根据这种方法,在TiCl(4)存在的情况下实现了席夫碱的有效形成。物质经反相色谱分离。在某些情况下,还可以通过在Chirobiotic T上进行手性色谱分离来分离异构体。在人重组黑皮质素受体上进行测试时,所有叔酰胺都表现出一定的结合亲和力。对于具有最高
-
Sulfur-controlled and rhodium-catalyzed formal (3 + 3) transannulation of thioacyl carbenes with alk-2-enals and mechanistic insights作者:Qiuyue Wu、Ziyang Dong、Jiaxi Xu、Zhanhui YangDOI:10.1039/d1ob00116g日期:——
A rhodium-catalyzed denitrogenative formal (3 + 3) transannulation of 1,2,3-thiadiazoles with alk-2-enals is achieved and a mechanistic investigation is performed, with an inverse KIE of 0.49 obtained.
表征谱图
-
氢谱1HNMR
-
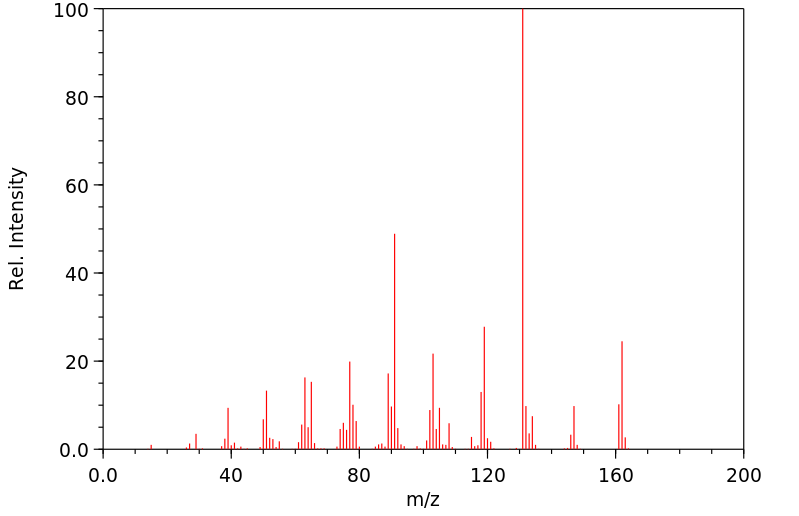
质谱MS
-
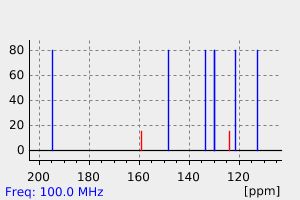
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
间三氟甲基肉桂醛
间三氟甲基肉桂醛
邻硝基肉桂醛
邻氯肉桂醛
邻氟肉桂醛
茚-2-甲醛
苯甲酸,2-[[2-羟基-3-(2,3,6,7-四氢-3,7-二甲基-2,6-二羰基-1H-嘌呤-1-基)丙基][3-(三氟甲基)苯基]氨基]-
肉桂醛
甲位戊基桂醛
对硝基肉桂醛
对甲基肉桂醛
对氟肉桂醛
反式肉桂醛
反式-肉桂醛
反式-alpha-甲基肉桂醛
反-4-氟肉桂醛
反-4-(二乙胺基)肉桂醛
厄洛替尼杂质46
亚苄基丙二醛
丁醛,4-氯-2-[氯(4-甲基苯基)亚甲基]-
α-甲基肉桂醛
α-甲基肉桂醛
α-溴代肉桂醛
α-氯代肉桂醛
α-己基肉桂醛
alpha-乙基肉桂醛
N-乙酰基-3-氨基-3-苯基-2-丙烯醛
8-溴-6-氯-2H-苯并吡喃-3-甲醛
6-羟基苯并吡喃-3-甲醛
6,8-二溴-2H-色烯-3-甲醛
5-甲氧基-2H-色烯-3-甲醛
5-氯-2-(氯苯基亚甲基)戊醛
4-羟基肉桂醛
4-硝基肉桂醛
4-甲氧基肉桂醛
4-甲氧基肉桂醛
4-溴肉桂醛
4-氯肉桂醛
4-叔-丁基-2-甲基肉桂醛
4-二甲基氨基肉桂醛
4-三氟甲氧基桂皮醛
3-苯基戊-2-烯醛
3-苯基壬-2-烯-4-炔醛
3-苯基-3-苯基硫代-2-丙烯醛
3-甲基-1H-茚-2-甲醛
3-溴肉桂醛
3-溴-3-苯基丙-2-烯醛
3-溴-3-(4-氯苯基)丙烯醛
3-氯肉桂醛
3-氯-3-苯基丙烯醛