2-溴-3-硝基吡啶 | 19755-53-4
分子结构分类
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:122-125 °C (lit.)
-
沸点:244.0±20.0 °C(Predicted)
-
密度:1.8727 (rough estimate)
-
稳定性/保质期:
如果按照规则使用和储存,则不会分解。避免接触氧化物。
计算性质
-
辛醇/水分配系数(LogP):1.7
-
重原子数:10
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:58.7
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xn
-
安全说明:S22,S26,S36,S36/37/39
-
危险类别码:R20/21/22,R36/37/38
-
WGK Germany:3
-
海关编码:2933399090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:在密封的贮藏器中,并将其存放在阴凉、干燥的地方。
SDS
模块 1. 化学品
产品名称: 2-Bromo-3-nitropyridine
修改号码: 5.3
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 2-溴-3-硝基吡啶
百分比: >98.0%(GC)
CAS编码: 19755-53-4
分子式: C5H3BrN2O2
2-溴-3-硝基吡啶 修改号码:5.3
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 微浅黄色-黄色
气味: 无资料
2-溴-3-硝基吡啶 修改号码:5.3
模块 9. 理化特性
pH: 无数据资料
熔点:
124°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂]
溶于: 热甲醇
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx), 溴化氢
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
2-溴-3-硝基吡啶 修改号码:5.3
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
制备方法与用途
性质
2-溴-3-硝基吡啶在常温常压下呈现灰白色至奶油色结晶粉末状,属于吡啶类衍生物,具有吡啶环的通用性质。
用途
2-溴-3-硝基吡啶可用作医药化学和有机合成中间体。在合成过程中,吡啶环上的硝基可以被还原成氨基;受吡啶环缺电子特性和硝基强吸电子特性的影响,结构中的溴原子可被亲核试剂如烷基胺或氧负离子进攻,从而进一步衍生化。
合成方法
将25毫升二甲基亚砜中溶解的48%氢溴酸(2.31毫升,20毫摩尔)溶液滴加到含有2-氨基-3-硝基吡啶(0.986克,5毫摩尔)和25毫升二甲基亚砜以及10毫摩尔亚硝酸钾的混合体系中。反应物在35℃下搅拌后,转移到含5克碳酸钾的100毫升冰水溶液中。随后,将反应混合物溶解于乙醚中,并用水洗涤。使用无水硫酸镁干燥后,在减压条件下除去有机溶剂,得到粗品4-溴二苯甲酮。通过柱层析进一步纯化,最终获得2-溴-3-硝基吡啶。
合成路线
反应信息
-
作为反应物:描述:参考文献:名称:通过硝基芳烃还原和亚胺水加氢一锅法构建四氢喹喔啉的无金属级联反应摘要:开发了一种无金属一锅级联工艺,包括还原硝基芳烃、环化以及用 B 2 cat 2和水对亚胺进行连续氢化,以直接从容易获得的 2-硝基苯胺或邻二硝基苯与 α-酮酯构建二氢喹喔啉酮和四氢喹喔啉和α-二酮。该策略以良好至优异的产率和对许多官能团的耐受性提供了各种二氢喹喔啉酮和四氢喹喔啉。通过控制实验和密度泛函理论(DFT)计算进行的机理研究表明,该级联过程涉及硝基芳烃的脱氧还原、美拉德反应和氢化生成二氢喹喔啉酮和四氢喹喔啉。该方法旨在减少对环境的影响,同时保持硝基芳烃还原和二氢喹喔啉酮和四氢喹喔啉合成的高产率和选择性。DOI:10.1039/d3nj05093a
-
作为产物:描述:参考文献:名称:新型卤代吡啶基硼酸和酯的合成。第2部分:2,4或5-卤代吡啶-3-基硼酸和酯摘要:本文描述了用于合成和5-卤代吡啶-3-基-硼酸酯的一般方法的新的2,4-隔离,或和4,7,10,13,15。这些化合物的制备考虑了使用n BuLi进行的区域选择性卤素-金属交换或区域选择性邻位交换-使用二异丙基氨基锂进行锂化,然后从适当的单或二卤代吡啶开始,用硼酸三异丙基酯淬灭。迄今为止研究的所有底物均提供单一的区域异构硼酸或酯产物。另外,已经发现这些化合物与一系列的芳基卤化物进行了Pd催化的偶联,并授权了一种制备新吡啶文库的策略。DOI:10.1016/s0040-4020(02)00283-1
文献信息
-
Efficient one-pot transformation of aminoarenes to haloarenes using halodimethylisulfonium halides generated in situ作者:Woonphil Baik、Wanqiang Luan、Hyun Joo Lee、Cheol Hun Yoon、Sangho Koo、Byeong Hyo KimDOI:10.1139/v05-026日期:2005.3.1
Halodimethylsulfonium halide 1, which is readily formed in situ from hydrohaloic acid and DMSO, is a good nucleophilic halide. This activated nucleophilic halide rapidly converts aryldiazonium salt prepared in situ by the same hydrohaloic acid and nitrite ion to aryl chlorides, bromides, or iodides in good yield. The combined action of nitrite ion and hydrohaloic acid in DMSO is required for the direct transformation of aromatic amines, which results in the production of aryl halides within 1 h. Substituted compounds with electron-donating or -withdrawing groups or sterically hindered aromatic amines are also smoothly transformed to the corresponding aromatic halides. The only observed by-product is the deaminated arene (usually <7%). The isolated aryldiazonium salts can also be converted to the corresponding aryl halides using 1. The present method offers a facile, one-step procedure for transforming aminoarenes to haloarenes and lacks the environmental pollutants that usually accompany the Sandmeyer reaction using copper halides. Key words: aminoarenes, haloarenes, halodimethylsulfonium halide, halogenation, amination.
卤二甲基亚砜卤化物1是一种良好的亲核卤化物,可在现场由氢卤酸和二甲亚砜形成。这种活化的亲核卤化物迅速将由相同的氢卤酸和亚硝酸根在现场制备的芳基重氮盐转化为芳基氯化物、溴化物或碘化物,收率较高。在DMSO中,亚硝酸根和氢卤酸的联合作用是直接转化芳香胺的必要条件,从而在1小时内产生芳基卤化物。带有电子给体或吸引基团或有立体位阻的芳香胺的取代化合物也可顺利转化为相应的芳香卤化物。观察到的唯一副产物是去氨基芳烃(通常<7%)。孤立的芳基重氮盐也可以使用1转化为相应的芳基卤化物。该方法提供了一种简便的、一步法的程序,用于将氨基芳烃转化为卤代芳烃,并且不伴随通常伴随使用铜卤化物进行桑迈尔反应的环境污染物。关键词:氨基芳烃,卤代芳烃,卤二甲基亚砜卤化物,卤化,胺化。 -
C−H Methylation of Iminoamido Heterocycles with Sulfur Ylides**作者:Prithwish Ghosh、Na Yeon Kwon、Saegun Kim、Sangil Han、Suk Hun Lee、Won An、Neeraj Kumar Mishra、Soo Bong Han、In Su KimDOI:10.1002/anie.202010958日期:2021.1.4The direct methylation of N‐heterocycles is an important transformation for the advancement of pharmaceuticals, agrochemicals, functional materials, and other chemical entities. Herein, the unprecedented C(sp2)‐H methylation of iminoamido heterocycles as nucleoside base analogues is described. Notably, trimethylsulfoxonium salt was employed as a methylating agent under aqueous conditions. A wide substrate
-
Highly active mesoionic chalcogenone zinc(<scp>ii</scp>) derivatives for C–S cross-coupling reactions作者:Moulali Vaddamanu、Kavitha Velappan、Ganesan PrabusankarDOI:10.1039/c9nj04440j日期:——of the first zinc(II) mesoionic chalcogenone complexes were isolated. Three mono nuclear zinc(II) chalcogenone complexes, [(L1)Zn(Cl)2(HOMe)] (1), [(L2)Zn(Cl)2(HOMe)] (3) and [(L2)Zn(Br)2(HOMe)] (4), and two dinuclear zinc complexes, [(L1)Zn(Br)(μ2-Br)]2 (2) and [(L3)Zn(Br)(μ2-Br)]2 (5), containing mesoionic thione and selone ligands were synthesized and characterised. These new complexes 1–5 represent第一个中离子较重的硫族元素酮,L 1 -L 3 [ L 1 = 1-(2-间苯二甲酰基)-3-甲基-4-苯基三唑啉-5-硒酮;L 2= 1-(2-均三甲苯基)-3-甲基-4-苯基三唑啉-5-硫酮;分离并表征L 3= 1-(苄基)-2-3(甲基)-4-苯基三唑啉-5-硒]。密度泛函理论用于深入了解中离子硫属元素酮的σ供体和π接受性质。使用这些新的配体,分离了一系列的第一个锌(II)介电硫属元素酮配合物。三种单核锌(II)硫属酮配合物,[(L 1)Zn(Cl)2(HOMe)](1),[(L 2)Zn(Cl)2(HOMe)](3)和[(L 2)Zn(Br)2(HOMe)](4)和两个双核锌配合物, [(大号1)的Zn(BR)(μ 2 -Br)] 2(2)和[(大号3)锌(BR)(μ 2 -Br)] 2(5),含介离子硫酮和selone配体合成和表征。这些新复合物1-5代表第一个在结构上表征的介离
-
Metal-Free Deoxygenation and Reductive Disilylation of Nitroarenes by Organosilicon Reducing Reagents作者:Argha Bhattacharjee、Hiromu Hosoya、Hideaki Ikeda、Kohei Nishi、Hayato Tsurugi、Kazushi MashimaDOI:10.1002/chem.201801972日期:2018.8.6A metal‐free deoxygenation and reductive disilylation of nitroarenes was achieved using N,N′‐bis(trimethylsilyl)‐4,4′‐bipyridinylidene (1) under mild and neutral reaction conditions, and a broad functional group tolerance was possible in this reaction. Mono‐deoxygenation, giving a synthetically valuable N,O‐bis(trimethylsilyl)phenylhydroxylamine (7 a) as a readily available and safe phenylnitrene source使用N,N'-双(三甲基甲硅烷基)-4,4'-联吡啶亚烷基(1)在温和和中性的反应条件下实现了硝基芳烃的无金属脱氧和还原二甲硅烷基化反应,并且该反应可能具有广泛的官能团耐受性。单脱氧可得到合成上有价值的N,O-双(三甲基甲硅烷基)苯羟胺(7 a),是一种容易获得且安全的硝基苯来源的苯基亚硝酸,双脱氧可得到N,N-双(三甲基硅烷基)苯胺8。改变1的量很容易控制反应温度以及加入二苯并噻吩(DBTP)。2-芳基硝基苯与1的反应通过N,O-双(三甲基甲硅烷基)苯基羟胺7的热解衍生的原位生成的亚苯基硝基苯胺生成相应的咔唑14,随后将其插入分子内CH。此外,分子内的N-N偶联反应将2,2'-二硝基联苯衍生物还原1,得到相应的苯并[ c ]喹啉。
-
Reduction of aromatic and aliphatic nitro groups to anilines and amines with hypophosphites associated with Pd/C作者:Marc Baron、Estelle Métay、Marc Lemaire、Florence PopowyczDOI:10.1039/c3gc37024k日期:——The reduction of aromatic and aliphatic nitro groups to anilines and amines is performed with good yield and selectivity in short reaction times. A mixture of sodium hypophosphite and phosphinic acid is used in the presence of a heterogeneous catalyst 2.5 mol% of Pd/C (5%) in a biphasic water/2-MeTHF system.
表征谱图
-
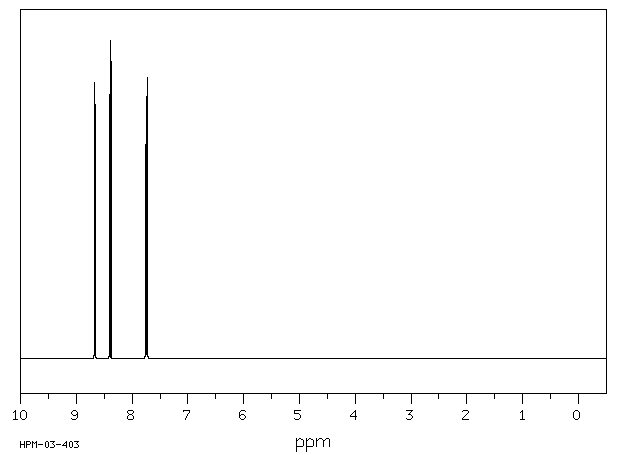
氢谱1HNMR
-
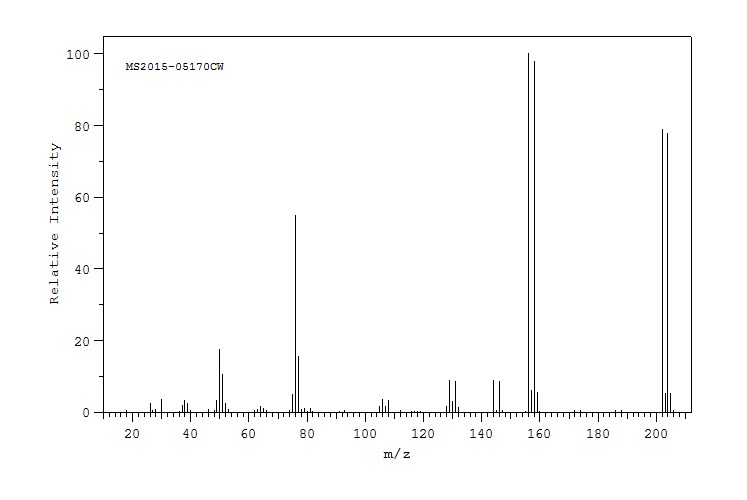
质谱MS
-
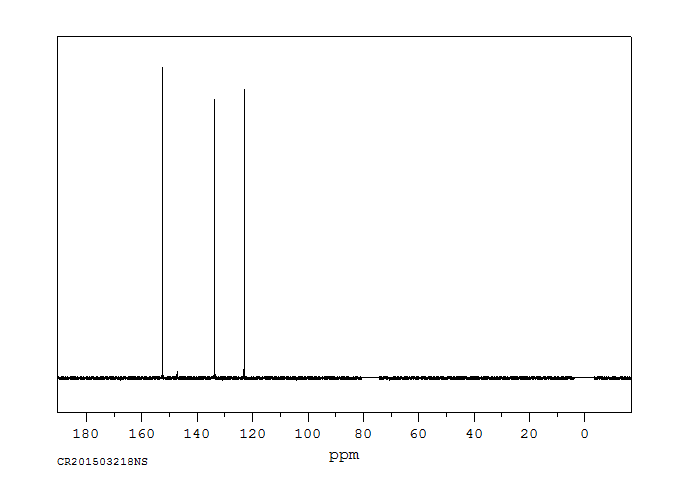
碳谱13CNMR
-
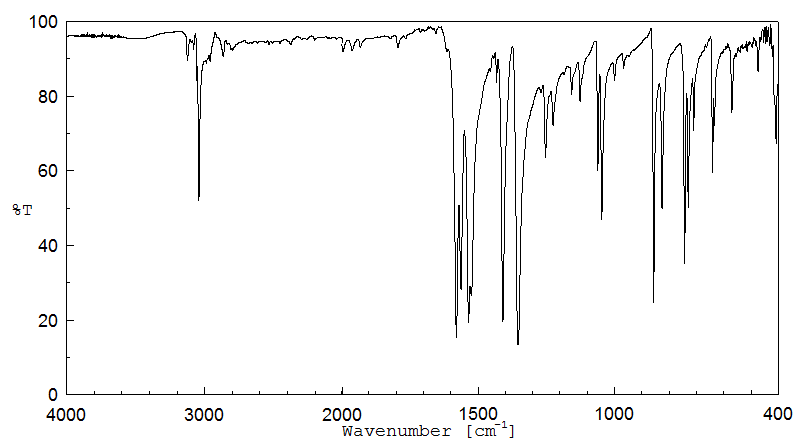
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息