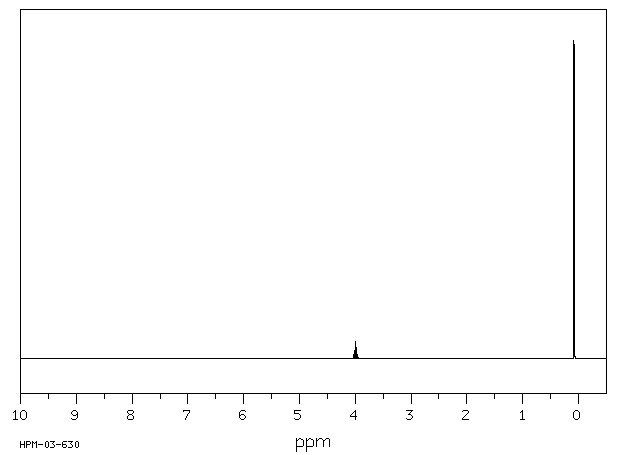
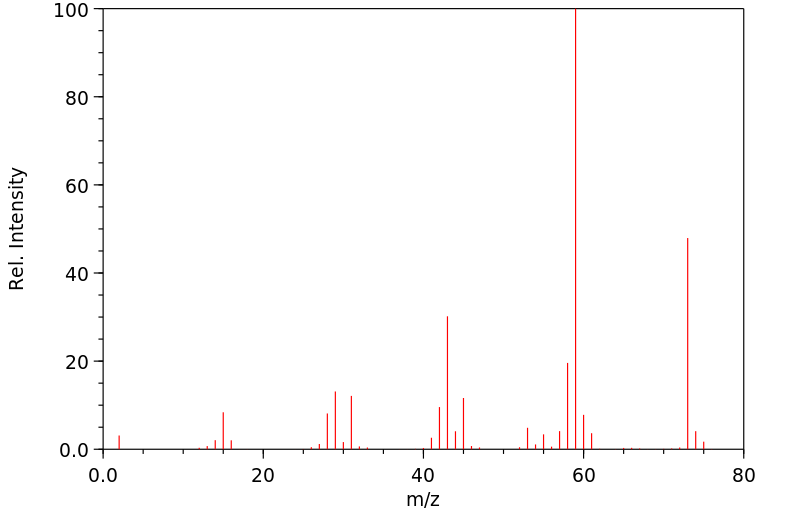
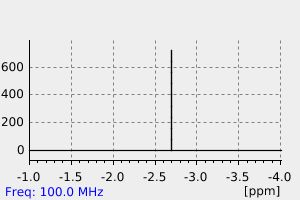
毒理性
大鼠LC50 > 5000 ppm/1小时
LC50 (rat) > 5,000 ppm/1hr
来源:Haz-Map, Information on Hazardous Chemicals and Occupational Diseases