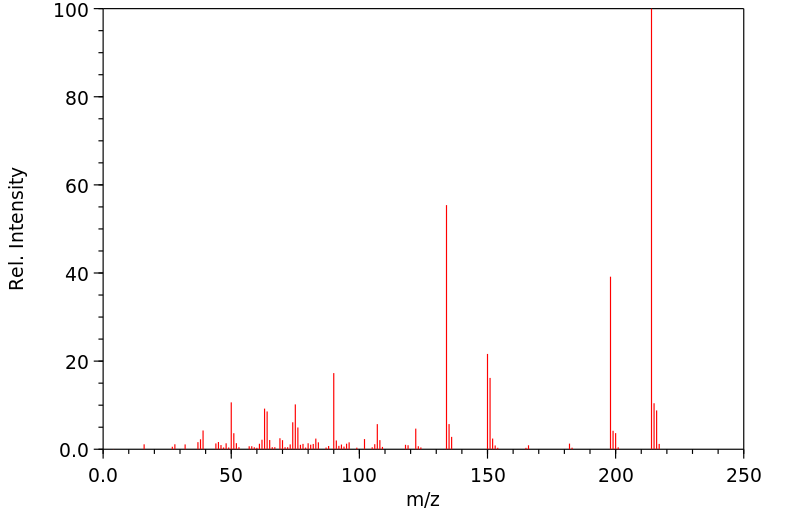
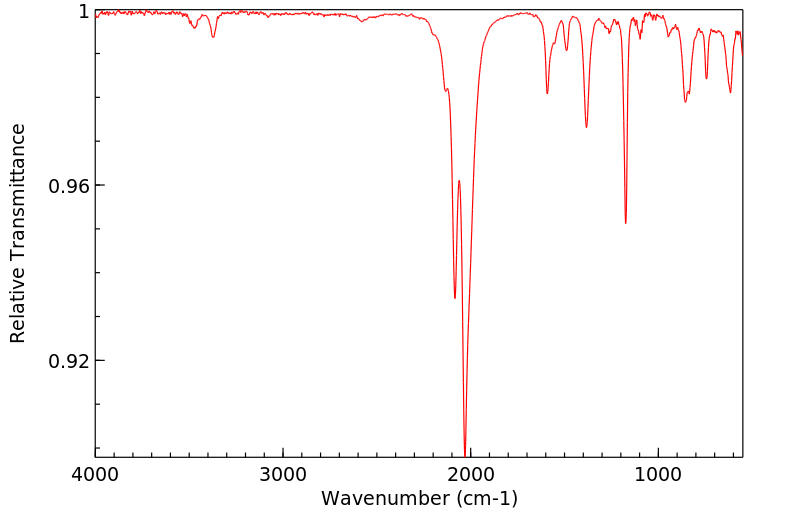
4-异硫氰基苯磺酰胺 | 51908-29-3
中文名称
4-异硫氰基苯磺酰胺
中文别名
4-异硫氰酸酯基苯磺酰胺
英文名称
4-isothiocyanatobenzene sulfonamide
英文别名
4-aminosulfonylphenyl isothiocyanate;4-Sulfamoyl-phenylisothiocyanat;4-Aminosulfonyl-phenyl-isothiocyanat;p-Aminosulfonyl-phenyl-isothiocyanat;4-Isothiocyanatobenzenesulfonamide
CAS
51908-29-3
化学式
C7H6N2O2S2
mdl
MFCD00079768
分子量
214.269
InChiKey
IMDUFDNFSJWYQT-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:210 °C
-
沸点:418.2±47.0 °C(Predicted)
-
密度:1.45±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.1
-
重原子数:13
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:113
-
氢给体数:1
-
氢受体数:5
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
安全说明:S26,S36/37/39
-
危险类别码:R20/21/22
-
危险品运输编号:UN 2811
-
海关编码:2935009090
-
储存条件:-20°C,保存在惰性气体中。
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 4-Isothiocyanatobenzenesulfonamide
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 4-Isothiocyanatobenzenesulfonamide
CAS number: 51908-29-3
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels, refrigerated.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C7H6N2O2S2
Molecular weight: 214.3
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides, sulfur oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 4-Isothiocyanatobenzenesulfonamide
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 4-Isothiocyanatobenzenesulfonamide
CAS number: 51908-29-3
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels, refrigerated.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C7H6N2O2S2
Molecular weight: 214.3
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides, sulfur oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
反应信息
-
作为反应物:描述:4-异硫氰基苯磺酰胺 在 potassium hydroxide 、 肼 作用下, 以 四氢呋喃 、 N,N-二甲基甲酰胺 为溶剂, 反应 6.5h, 生成 4-((5-amino-1H-1,2,4-triazol-3-yl)amino)benzenesulfonamide参考文献:名称:恶性疟原虫蛋白激酶5(PK5)抑制剂的计算机筛选和评估摘要:使用无偏方法对35万种市售化合物进行了计算机筛选,以鉴定与恶性疟原虫蛋白激酶5(Pf PK5)ATP结合位点结合的潜在疟疾抑制剂。P f PK5是一种细胞周期蛋白依赖性激酶样蛋白,与人细胞周期蛋白依赖性激酶2(Hs CDK2)具有高度序列相似性,但其在细胞周期调控中的确切作用尚不清楚。在对得分最高的化合物进行二维指纹图谱分析后,根据其结构多样性对182种候选化合物进行了生化测试的优先级排序。对这些化合物的评估表明,135与Pf PK5的结合程度与已知Pf相似或更好。PK5抑制剂,证实该文库富含Pf PK5结合化合物。分别选择了先前报道的三唑二胺Hs CDK2抑制剂和筛选出的4-甲基伞形酮进行类似物研究。这项研究的结果强调优化难以兼顾Pf的用于PK5亲和力和结合选择性Pf的PK5超过其最接近人类同源HS CDK2。我们的方法能够通过适度的筛选活动发现几种新的Pf PK5结合化合物,并揭示了第一个具有改善的PfDOI:10.1002/cmdc.201800625
-
作为产物:描述:参考文献:名称:作为选择性碳酸酐酶抑制剂的新型苯磺酰胺吡唑和 1,2,4-噻二唑摘要:合成了两个系列,包括 20 种带有硫脲基连接的吡唑8和氨基-1,2,4-噻二唑10的新型苯磺酰胺,并作为人碳酸酐酶 (hCA) 抑制剂针对亚型 I 和 II 以及肿瘤相关亚型 IX 和十二.还针对亚型 hCA I、II 和 XII 进行了一些有效衍生物( 8a 、 8c 、 10a和10c )的分子模型研究。这两个有前景的系列化合物都是使用市售的甲基酮和磺胺作为起始原料合成的。有趣的是,本文还报道了一种使用3-氨基异恶唑和4-异硫氰酸苯磺酰胺作为反应物合成氨基-1,2,4-噻二唑10的新方法。所有新合成的化合物的活性特征表明,与硫脲基连接的吡唑8相比,氨基连接的 1,2,4-噻二唑10是胞质异构体 hCA I 更好的抑制剂。此外,hCA II 几乎被所有新合成的磺酰胺类药物强烈抑制,而与标准药物乙酰唑胺相比,所有化合物作为 hCA IX 和 XII 抑制剂的效果较差。然而,就选择性而言,化合物8e被发现是hCADOI:10.1002/ardp.202100241
文献信息
-
Carbonic Anhydrase Inhibitors: Water-Soluble 4-Sulfamoylphenylthioureas as Topical Intraocular Pressure-Lowering Agents with Long-Lasting Effects作者:Angela Casini、Andrea Scozzafava、Francesco Mincione、Luca Menabuoni、Marc A. Ilies、Claudiu T. SupuranDOI:10.1021/jm001051+日期:2000.12.1A series of sulfonamides has been obtained by reaction of 4-isothiocyanatobenzenesulfonamide with amines, amino acids, and oligopeptides. The new thiourea derivatives showed strong affinities toward isozymes I, II, and IV of carbonic anhydrase (CA, EC 4.2.1.1). In vitro inhibitory power was good (in the low-nanomolar range) for the derivatives of beta-phenylserine and alpha-phenylglycine, for those通过4-异硫氰酸根合苯磺酰胺与胺,氨基酸和寡肽的反应获得了一系列磺酰胺。新的硫脲衍生物对碳酸酐酶的同功酶I,II和IV具有很强的亲和力(CA,EC 4.2.1.1)。对于β-苯基丝氨酸和α-苯基甘氨酸的衍生物,掺入羟基和巯基氨基酸(Ser,Thr,Cys,Met),疏水性氨基酸(Val, Leu,Ile),芳香族氨基酸(Phe,His,Trp,Tyr,DOPA)和二羧酸氨基酸,以及二/三/四肽等。这种CA抑制剂主要以(羧酸盐)钠盐形式显示出非常好的水溶性(在2-3%范围内),所得溶液的pH值为6.5-7.0。这些制剂中的某些(例如Ser,β-Ph-Ser,Leu,Asn等的衍生物)以2%的水溶液直接局部施用到降压/青光眼兔眼中时,可大大降低眼压(IOP) 。有趣的是,并非本研究中设计的所有功能强大的CA抑制剂都显示出局部IOP降低作用(例如,Cys和Lys衍生物,缺乏这种特性),而Pro,
-
Carbonic anhydrase inhibitors. Synthesis and inhibition of cytosolic/tumor-associated carbonic anhydrase isozymes I, II, and IX with boron-containing sulfonamides, sulfamides, and sulfamates: Toward agents for boron neutron capture therapy of hypoxic tumors作者:Jean-Yves Winum、Alessandro Cecchi、Jean-Louis Montero、Alessio Innocenti、Andrea Scozzafava、Claudiu T. SupuranDOI:10.1016/j.bmcl.2005.04.058日期:2005.7against hCA IX in the range of 7.3-89nM, respectively. As hypoxic tumors highly overexpress CA IX, the design of boron-containing inhibitors with high affinity for the tumor-associated CA isozymes may lead to important advances in boron neutron capture therapy (BNCT) applications targeting such tumors, which are non-responsive to both classical chemo- and radiotherapy.据报道,含硼的碳酸酐酶(CA,EC 4.2.1.1)抑制剂库包括磺酰胺,磺酰胺和氨基磺酸盐。通过4-羧基-/氨基-/羟基-苯基硼酸频哪醇酯与氨基/异硫氰酸根基取代的芳族/杂芳族磺酰胺的衍生化反应或与氨磺酰氯的氨磺酰化反应,可以合成新化合物。已经测定了这些新衍生物对三种生理相关的CA同工酶(胞质CA I和II,以及跨膜的肿瘤相关同工酶IX)的抑制作用。在磺酰胺,氨基磺酸盐和磺酰胺中均检测到有效的抑制剂。新化合物针对人同工酶hCA I的抑制常数范围为34-94nM,针对hCA II的抑制常数范围为3.1-48nM,针对hCA IX的抑制常数范围为7。分别为3-89nM。由于缺氧性肿瘤高度表达CA IX,因此设计与肿瘤相关的CA同工酶具有高亲和力的含硼抑制剂可能会导致针对此类肿瘤的硼中子俘获疗法(BNCT)应用的重要进展,而这对两种肿瘤均无反应经典的化学和放射疗法。
-
Thiazole compounds and pharmaceutical compositions for inhibiting protein kinases and methods for their use申请人:Agouron Pharmaceuticals, Inc.公开号:US20020025976A1公开(公告)日:2002-02-28Diaminothiazole compounds that modulate and/or inhibit the activity of certain protein kinases are described. These compounds and pharmaceutical compositions containing them are capable of mediating tyrosine kinase signal transduction in order to modulate and/or inhibit unwanted cell proliferation. The invention is also directed to the therapeutic or prophylactic use of pharmaceutical compositions containing such compounds, and to methods of treating cancer as well as other disease states associated with unwanted angiogenesis and/or cellular proliferation, such as diabetic retinopathy, neovascular glaucoma, rheumatoid arthritis, and psoriasis, by administering effective amounts of such compounds.
-
In-Vitro Anticancer Evaluation of Some Novel Thioureido-Benzensulfonamide Derivatives作者:Mostafa Ghorab、Mansour Alsaid、Mohammed Al-Dosari、Marwa El-Gazzar、Ahmed ArbabDOI:10.3390/molecules21040409日期:——A novel series of sulfonamide derivatives (14 compounds) bearing thiourea moieties were efficiently synthesized and evaluated for their possible in vitro anticancer activity against four human tumor cell lines. The results indicated that compound 6 was the most potent, showing effectiveness on all the tested cell lines. Compounds 7 and 10 also showed promising results.
-
[EN] TRIAZOLE COMPOUNDS AS ANTIVIRALS<br/>[FR] COMPOSÉS TRIAZOLES EN TANT QU'ANTIVIRAUX申请人:HOFFMANN LA ROCHE公开号:WO2014006066A1公开(公告)日:2014-01-09The present invention discloses compounds of Formula I: wherein the variables in Formula I are defined as described herein. Also disclosed are pharmaceutical compositions containing such compounds and methods for using the compounds of Formula I in the prevention or treatment of HCV infection.本发明公开了公式I的化合物:其中公式I中的变量定义如本文所述。还公开了包含此类化合物的药物组合物以及使用公式I化合物预防或治疗HCV感染的方法。
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫