(2,2-二氯-1-苯乙烯基)苯 | 2779-69-3
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
保留指数:1754
计算性质
-
辛醇/水分配系数(LogP):5.4
-
重原子数:16
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2903999090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 2-chloro-1,1-diphenylethene 4541-89-3 C14H11Cl 214.694 1,1-二苯乙烯 1,1-Diphenylethylen 530-48-3 C14H12 180.249 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-chloro-1,1-diphenylethene 4541-89-3 C14H11Cl 214.694 1,1-双-(4-溴苯基)-2,2-二氯乙烯 1,1-dichloro-2,2-bis(4'-bromophenyl)ethylene 21655-73-2 C14H8Br2Cl2 406.932
反应信息
-
作为反应物:描述:参考文献:名称:The Halogenation of Unsymmetrical Diphenylethane1摘要:DOI:10.1021/ja01861a037
-
作为产物:描述:参考文献:名称:Li, Nippon Kagaku Kaishi/Journal of the Chemical Society of Japan, 1943, vol. 64, p. 1399,1404摘要:DOI:
文献信息
-
The Influence of Growth Hormone Deficiency, Growth Hormone Replacement Therapy, and Other Aspects of Hypopituitarism on Fracture Rate and Bone Mineral Density作者:Christian Wüster、Roger Abs、Bengt-Åke Bengtsson、Helge Bennmarker、Ulla Feldt-Rasmussen、Elizabeth Hernberg-Ståhl、John P. Monson、Bjørn Westberg、Patrick WiltonDOI:10.1359/jbmr.2001.16.2.398日期:——To assess the influence of factors affecting fracture risk and bone density in adult hypopituitary patients with growth hormone deficiency (GHD), data from a large‐scale pharmacoepidemiological survey (the Pharmacia & Upjohn International Metabolic Database [KIMS]) were analyzed and compared with data from a control population (the European Vertebral Osteoporosis Study [EVOS]). The KIMS group consisted of 2084 patients (1112 men and 972 women) with various types of pituitary disease and EVOS consisted of 1176 individuals (581 men and 595 women). Fracture and bone mineral density (BMD) data were available from 2024 patients from the KIMS group and 392 patients from EVOS. The prevalence of fractures in patients with hypopituitarism was 2.66 times that in the non‐GH‐deficient EVOS population. Adult‐onset hypopituitarism with GHD was associated with a higher fracture risk than childhood‐onset disease, and patients with isolated GHD had a similar prevalence of fractures to those with multiple pituitary hormone deficiencies. Hormonal replacement therapy with L‐thyroxine, glucocorticoids, and sex steroids did not affect the risk of fracture in KIMS patients. In addition, fracture rates in KIMS were independent of body mass index (BMI) and the country of origin. However, smoking was associated with a higher fracture rate in this group. In summary, this is the first large‐scale analysis to support the hypothesis of an increased fracture risk in adult patients with hypopituitarism and GHD. This increased risk appears to be attributable to GHD alone, rather than to other pituitary hormone deficiencies or to their replacement therapy.为了评估影响成年垂体功能减退症患者骨折风险和骨密度的因素,特别是生长激素缺乏症(GHD)的影响,我们分析了一项大规模药物流行病学调查(法玛西亚与安万特国际代谢数据库[KIMS])的数据,并与对照人群(欧洲椎骨骨质疏松症研究[EVOS])的数据进行了比较。KIMS组包括2084名患者(1112名男性和972名女性),他们患有各种类型的垂体疾病,而EVOS组包括1176名个体(581名男性和595名女性)。从KIMS组的2024名患者和EVOS组的392名患者中获得了骨折和骨矿物质密度(BMD)数据。垂体功能减退症患者的骨折发生率是GH非缺乏的EVOS人群的2.66倍。与儿童期发病的疾病相比,成年期发病的垂体功能减退症伴GHD与更高的骨折风险相关,而孤立性GHD患者的骨折发生率与多重垂体激素缺乏症患者的骨折发生率相似。使用L-甲状腺素、糖皮质激素和性激素的激素替代疗法并未影响KIMS患者的骨折风险。此外,KIMS中的骨折率与体重指数(BMI)和原籍国无关。然而,吸烟与该组较高的骨折率相关。总之,这是首次大规模分析支持成年垂体功能减退症和GHD患者骨折风险增加的假设。这种增加的风险似乎仅归因于GHD,而非其他垂体激素缺乏或其替代疗法。
-
Kinetic and quantum chemical studies of the mechanism of dehydrochlorination of 2,2-diaryl-1,1,1-trichloroethanes with nitrite ions作者:V. N. Kazin、M. B. Kuzhin、A. V. Sirik、E. A. GuzovDOI:10.1134/s1070428016090049日期:2016.9The E2 mechanism has been proposed for the dehydrochlorination of 2,2-diaryl-1,1,1-trichloroethanes with nitrite ion, leading to 2,2-diaryl-1,1-dichloroethenes, on the basis of experimental kinetic study and quantum chemical simulation.
-
Photocycloelimination of α,α-dichlorocyclobutanones作者:Jailall Ramnauth、Edward Lee-RuffDOI:10.1139/v99-110日期:1999.7.1
Direct irradiation of a series of α,α-dichlorocyclobutanones in benzene solutions results in photocycloelimination to give 1,1-dichloroalkenes in yields ranging from 30-65%. The α,α-dichlorocyclobutanones were formed in good yields from the [2+2] cycloaddition of the terminal olefins with dichloroketene. This two-step sequence formally represents a "metathesis" of two olefinic functions and provides an easy access to functionalized 1,1-dichloroalkenes. Irradiation of the dichlorocyclobutanones in the solid state led to poor yields of 1,1-dichloroalkenes and polymeric mixtures, however, photoreactions performed in zeolites gave similar yields as those run in benzene solutions.Key words: dichlorocyclobutanones, dichloroalkenes, olefin metathesis, photocycloelimination.
-
Synthesis of annulated oligothiophenes作者:V. G. Nenajdenko、E. S. Balenkova、K. Y. Chernichenko、S. S. VshivenkoDOI:10.1007/s11172-012-0188-1日期:2012.7Reactions of 1,1-di(2-naphthyl)-2,2-dichloroethene and 1,1-di(2-benzothienyl)-2,2-dichloroethene with sulfur at 220–225 °C resulted in hitherto unknown oligothiophenes. Tetrathio[6]helicene was synthesized from 1,1-di(3-benzothienyl)-2,2-dichloroethene. Preparative pathway to helicene involving intramolecular ring closure of dithiol derived from 1,1-di-(3-benzothienyl)-2,2-dichloroethene was developed as an alternative to high temperature synthesis.
-
Ramberg-Bäcklund rearrangement vs. β-Elimination of haloform from trichloro and trifluoromethyl sulfones作者:Samuel Braverman、Yossi ZafraniDOI:10.1016/s0040-4020(97)10404-5日期:1998.2A new and convenient method for the preparation of trichloro and trifluoromethanesulfinates is described. These esters readily undergo rearrangement to the corresponding sulfones at room temperature, in high yields. In contrast to trichloromethyl sulfoxides which undergo base-induced β-elimination of chloroform to sulfines, the corresponding sulfones undergo an unusually facile Ramberg-Bäcklund rearrangement
表征谱图
-
氢谱1HNMR
-
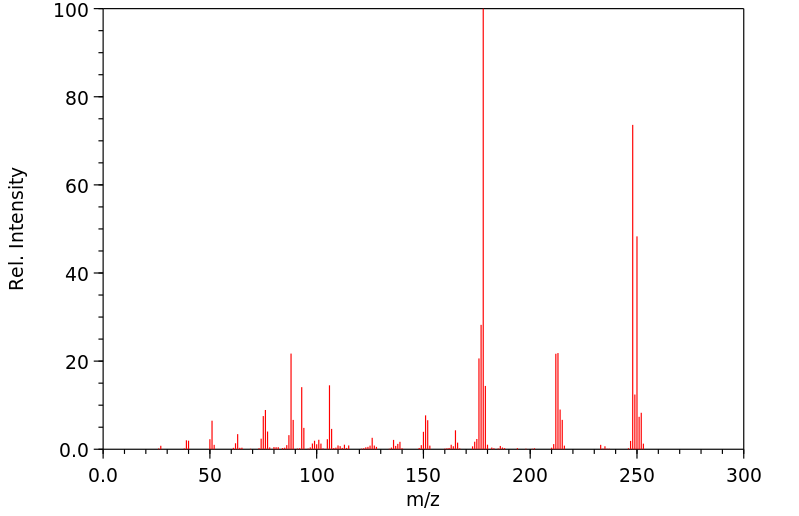
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息