苯甲基磺酰氟 | 329-98-6
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:92-95 °C
-
沸点:112 °C16 mm Hg(lit.)
-
密度:0.797 g/mL at 20 °C
-
闪点:222 °F
-
溶解度:无水溶剂(乙醇、甲醇和2-丙醇):200 mM储备液在4°C下可稳定数月。
计算性质
-
辛醇/水分配系数(LogP):1.3
-
重原子数:11
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.142
-
拓扑面积:42.5
-
氢给体数:0
-
氢受体数:3
ADMET
安全信息
-
TSCA:T
-
危险等级:8
-
危险品标志:T
-
安全说明:S16,S26,S28A,S36/37/39,S45,S7
-
危险类别码:R34,R25
-
WGK Germany:3
-
海关编码:2904909090
-
危险品运输编号:UN 3261 8/PG 2
-
危险类别:8
-
RTECS号:XT8050000
-
包装等级:III
-
危险标志:GHS05,GHS06
-
危险性描述:H301,H314
-
危险性防范说明:P260,P280,P301 + P310 + P330,P303 + P361 + P353,P304 + P340 + P310,P305 + P351 + P338 + P310
-
储存条件:室温保存。
SDS
| Name: | alpha-Toluenesulfonyl fluoride 99% Material Safety Data Sheet |
| Synonym: | Phenylmethylsulfonyl fluoride; PMSF; Benzenemethanesulfonyl fluorid |
| CAS: | 329-98-6 |
Synonym:Phenylmethylsulfonyl fluoride; PMSF; Benzenemethanesulfonyl fluorid
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 329-98-6 | alpha-Toluenesulfonyl fluoride | 99.0 | 206-350-2 |
Risk Phrases: 34
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Causes burns.Moisture sensitive.Corrosive.
Potential Health Effects
Eye:
Causes eye burns. Contact with eyes may cause severe irritation, and possible eye burns.
Skin:
Causes skin burns.
Ingestion:
May cause severe and permanent damage to the digestive tract. Causes gastrointestinal tract burns.
Inhalation:
May cause severe irritation of the respiratory tract with sore throat, coughing, shortness of breath and delayed lung edema. Causes chemical burns to the respiratory tract.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately. Do NOT allow victim to rub eyes or keep eyes closed.
Extensive irrigation with water is required (at least 30 minutes).
Skin:
Get medical aid immediately. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Wash clothing before reuse. Destroy contaminated shoes.
Ingestion:
Do not induce vomiting. If victim is conscious and alert, give 2-4 cupfuls of milk or water. Never give anything by mouth to an unconscious person. Get medical aid immediately.
Inhalation:
Get medical aid immediately. Remove from exposure and move to fresh air immediately. If breathing is difficult, give oxygen. Do NOT use mouth-to-mouth resuscitation. If breathing has ceased apply artificial respiration using oxygen and a suitable mechanical device such as a bag and a mask.
Notes to Physician:
Antidote: None reported.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Do NOT use water directly on fire. Use foam, dry chemical, or carbon dioxide.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up, then place into a suitable container for disposal. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Do not get in eyes, on skin, or on clothing. Keep container tightly closed. Do not ingest or inhale. Use with adequate ventilation. Discard contaminated shoes.
Storage:
Keep container closed when not in use. Store in a cool, dry, well-ventilated area away from incompatible substances. Corrosives area. Keep containers tightly closed.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate general or local exhaust ventilation to keep airborne concentrations below the permissible exposure limits.
Exposure Limits CAS# 329-98-6: United States OSHA: 2.5 mg/m3 TWA (as F) (listed under Fluorides Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Needles
Color: white
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 92.00 - 94.00 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water: hydrolysis
Specific Gravity/Density:
Molecular Formula: C7H7FO2S
Molecular Weight: 174.19
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, exposure to moist air or water.
Incompatibilities with Other Materials:
Strong oxidizing agents, strong bases, moisture.
Hazardous Decomposition Products:
Carbon monoxide, oxides of sulfur, carbon dioxide, hydrogen fluoride gas.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 329-98-6: XT8040000 LD50/LC50:
CAS# 329-98-6: Oral, mouse: LD50 = 200 mg/kg.
Carcinogenicity:
alpha-Toluenesulfonyl fluoride - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: CORROSIVE SOLID, N.O.S.*
Hazard Class: 8
UN Number: 1759
Packing Group: II
IMO
Shipping Name: CORROSIVE SOLID, N.O.S.
Hazard Class: 8
UN Number: 1759
Packing Group: II
RID/ADR
Shipping Name: CORROSIVE SOLID, N.O.S.
Hazard Class: 8
UN Number: 1759
Packing group: II
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: C
Risk Phrases:
R 34 Causes burns.
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 329-98-6: No information available.
Canada
CAS# 329-98-6 is listed on Canada's DSL List.
CAS# 329-98-6 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 329-98-6 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
PMSF(苯甲基磺酰氟,对苯二酚磺酰氟)是一种不可逆的丝氨酸/半胱氨酸蛋白酶抑制剂。
靶点| Target | Value |
|---|---|
| 胰蛋白酶 | |
| 胰凝乳蛋白酶 |
PMSF可以迅速地抑制从人胰腺纯化的胰凝乳蛋白酶的活性,对人的胰蛋白酶影响较弱。此外,它也可以迅速地抑制人红细胞中的胰腺胆碱酯酶。
研究发现,2 mM PMSF即可几乎完全抑制豚鼠回肠中卡巴胆碱激活的肌糖形成磷脂酰肌醇(PI),但对甲基激活的途径没有影响。PMSF还能够短暂地抑制由卡巴胆碱和钾刺激产生的肌肉收缩。
此外,PMSF还能抑制布氏锥虫体内乙醇胺磷酸添加到糖基磷脂酰肌醇(GPI)中间产物的形成以及布氏锥虫血液中GPI中间产物的肌糖残基的酰化作用。它能抑制布氏锥虫前循环形式的乙醇胺磷酸的添加和肌糖的酰化,但不能抑制哺乳动物HeLa细胞中的这一过程。PMSF是鼠源乙酰胆碱酯酶的最佳抑制剂,即使用8倍量的BSF,其抑制效果仍比PMSF低6倍。
体内研究向Sprague-Dawley大鼠腹腔内注射PMSF能产生剂量依赖性的止痛效果。在大鼠体内,PMSF显著提升了β-内啡肽(END)的镇痛效果。小鼠腹腔内注射PMSF可产生类似大麻素的效果,表现为抗伤害、降低体温及保持不动性,其ED50分别为86 mg/kg, 224 mg/kg 和 206 mg/kg。
用低剂量(30 mg/kg)的PMSF预处理能够增强大麻素对甩尾反应(抗伤害效应)、自发活动性和移动性的作用,效果分别是原来的效果的5倍、10倍和8倍。在鸡SPG前12小时使用PMSF处理可以完全防御迟发性神经毒性(OPIDN),而在SPG后4小时使用则会增强神经毒性的效应。
注射1 或 10 mg/kg PMSF可预处理抑制三邻甲苯基磷酸酯(TOCP)诱导的神经丝(NF)退化,并预防母鸡有机磷酸酯诱导的延迟性神经病(OPIDN)。PMSF通过抑制脂肪酸酰胺水解酶活性,能够抑制ICR小鼠的Δ(9)- 四氢大麻酚(THC)或大麻素(AEA)的典型拟大麻效应。
用途可有效抑制丝氨酸蛋白酶如胰蛋白酶(trypsin)和胰凝乳蛋白酶(chymotrypsin),也能抑制半胱氨酸蛋白酶和乙酰胆碱酯酶,有效浓度为0.1-1 mM。
上下游信息
反应信息
-
作为反应物:参考文献:名称:通过磺酰氟和三甲基甲硅烷基叠氮化物的路易斯碱活化合成磺酰叠氮化物摘要:描述了通过路易斯碱活化将磺酰氟有效转化为磺酰叠氮化物的协议。原位生成的磺酰叠氮化物是有效的重氮转移剂,以优异的产率提供重氮化合物和初级叠氮化物。DOI:10.1055/s-0035-1561626
-
作为产物:参考文献:名称:Flash vacuum pyrolysis of stabilised phosphorus ylides. Part 12.1 Extrusion of Ph3P from sulfonyl ylides and reactivity of the resulting sulfonyl carbenes摘要:已制备十二种磺酰稳定的磷亚叶立德,并研究了它们在600 °C下的快速真空热解行为。带有芳基磺酰取代基的例子会失去Ph3PO,产生难以处理的产物,而带有芳基甲基磺酰取代基的则分别失去Ph3P和SO2,生成与磺酰烯烃中间体一致的产物。对每个系列中一种亚叶立德的X射线结构测定显示,第一种情况下P–O之间的非键合相互作用更为显著,这为不同的热反应性提供了一些解释。DOI:10.1039/a707948f
-
作为试剂:描述:参考文献:名称:微生物对映选择性去除N-苄氧羰基氨基保护基。摘要:为了使N-碳苯甲氧基-1-氨基酸(Cbz-AA)和相关化合物脱保护,通过以Cbz-1-Glu作为唯一氮源的富集培养从土壤中选择了一系列微生物。两种节杆菌属的冻干全细胞制剂。在Cbz-Glu或Cbz-Gly上生长的菌株显示出高裂解活性。已经优化了水解条件,并且获得了几种Cbz-dl-氨基酸的定量对映选择性脱保护,以及几种合成氨基化合物的N-氨基甲酸酯衍生物的脱保护。该方法的应用描述了以高收率和高光学纯度制备Cbz-d-烯丙基甘氨酸和1-烯丙基甘氨酸的方法。DOI:10.1016/j.molcatb.2012.03.005
文献信息
-
Modulators (inhibitors/ activators) of histone acetyltransferases申请人:Kundu Kumar Tapas公开号:US20060167107A1公开(公告)日:2006-07-27Disclosed are compounds of the formulae: and method of using the compounds to treat cancer, AIDS, HIV infection, and asthma.揭示了以下式的化合物: 以及使用这些化合物治疗癌症、艾滋病、HIV感染和哮喘的方法。
-
AKT INACTIVATION BY TOCOPHERYL DERIVATIVES申请人:Chen Ching-Shih公开号:US20140031388A1公开(公告)日:2014-01-30Anticancer compounds according to formula I are described herein. wherein R 1 , R 2 , R 3 and R 4 are selected from H, CH 3 , OH, SH, OCH 3 , NHR′, halogen, CF 3 , N-linked pyrrolidine, and SO 2 NHR′, or any combination thereof; R 5 is an alkyl, alkenyl, or alkaryl group including from 4 to 11 carbons, X is selected from CH 2 , CHOH, C═O, S═O, O═S═O, and an oxetane ring, Y is selected from CH 2 , O, and NH, and R′ is a H, aryl, or a lower alkyl group, or pharmaceutically acceptable salts thereof. The compounds have been shown to facilitate site-specific dephosphorylation of Akt at Ser-473, thereby inactivating Akt and decreasing dysregulation of Akt signaling that can occur in cancer cells.
-
Dual Pharmacophores - PDE4-Muscarinic Antagonistics申请人:Callahan James Francis公开号:US20090203657A1公开(公告)日:2009-08-13The present invention is directed to novel compounds of Formula (I) and pharmaceutically acceptable salts thereof, pharmaceutical compositions and their use as dual chromaphores having inhibitory activity against PDE4 and muscarinic acetylcholine receptors (mAChRs), and thus being useful for treating respiratory diseases.本发明涉及具有式(I)的新化合物及其药用盐,药物组合物及其用作对PDE4和肌胆碱受体(mAChRs)具有抑制活性的双色素,因此可用于治疗呼吸道疾病。
-
[EN] DUAL PHARMACOPHORES - PDE4-MUSCARINIC ANTAGONISTICS<br/>[FR] PHARMACOPHORES DUALS, ANTAGONISTES DES RÉCEPTEURS MUSCARINIQUES ET INHIBITEURS DE L'ACTIVITÉ PDE4申请人:GLAXO GROUP LTD公开号:WO2009100169A1公开(公告)日:2009-08-13The present invention is directed to novel compounds of Formula's (I) - (VI), and pharmaceutically acceptable salts thereof, pharmaceutical compositions and their use in therapy, for example as inhibitors of phosphodiesterase type IV (PDE4) and as antagonists of muscarinic acetylcholine receptors (mAChRs), in the treatment of and/or prophylaxis of respiratory diseases, including inflammatory and/or allergic diseases such as chronic obstructive pulmonary disease (COPD), asthma, rhinitis (e.g. allergic rhinitis), atopic dermatitis or psoriasis.
-
[EN] INHIBITORS<br/>[FR] INHIBITEURS申请人:BIONOMICS LTD公开号:WO2015123722A1公开(公告)日:2015-08-27This invention relates to compounds of the formula (I):The invention also relates to processes for the preparation of the compound of the formula (I), pharmaceutical agents or compositions containing the compound or a method of using the compound for the treatment of proliferative diseases, such as cancer.这项发明涉及式(I)的化合物。该发明还涉及制备式(I)化合物的方法,含有该化合物的药物或组合物,或者使用该化合物治疗增殖性疾病,如癌症的方法。
表征谱图
-
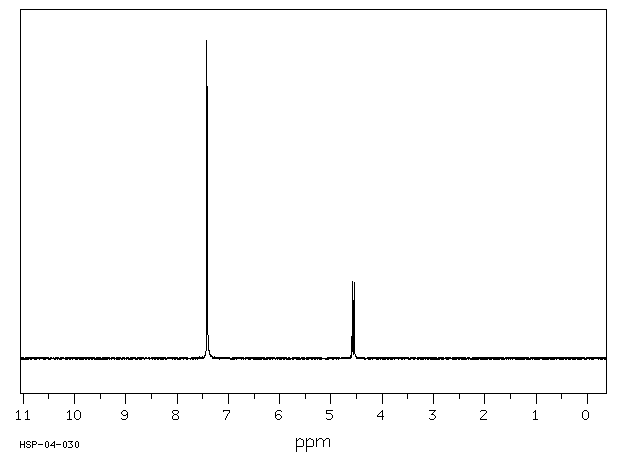
氢谱1HNMR
-
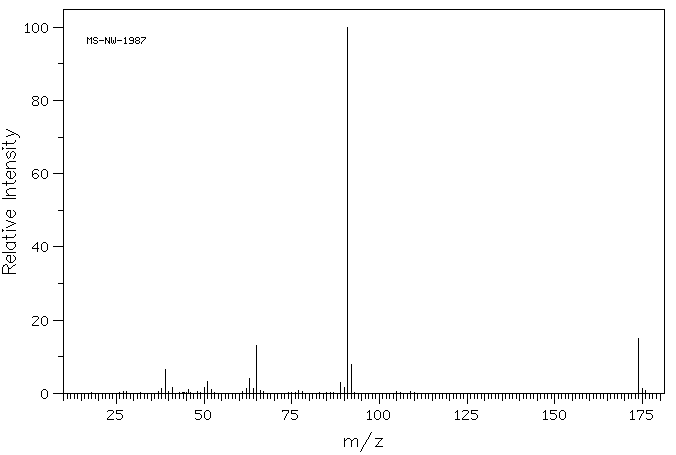
质谱MS
-
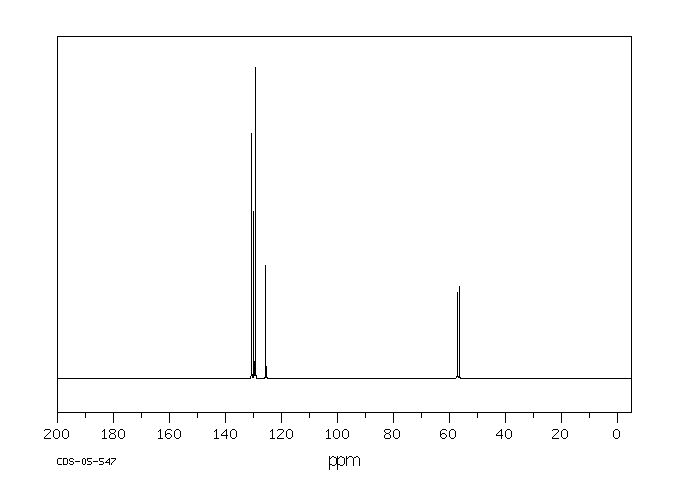
碳谱13CNMR
-
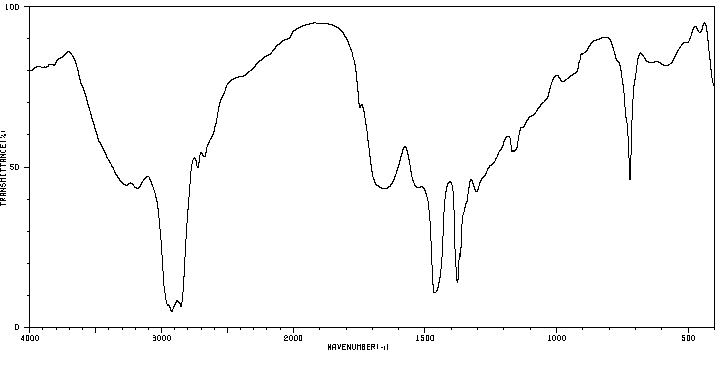
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息