苄基肼 | 555-96-4
中文名称
苄基肼
中文别名
苄肼
英文名称
Benzylhydrazine
英文别名
——
CAS
555-96-4
化学式
C7H10N2
mdl
MFCD00869518
分子量
122.17
InChiKey
NHOWLEZFTHYCTP-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:112 °C
-
沸点:217.6°C (rough estimate)
-
密度:1.0343 (rough estimate)
-
溶解度:8.18 M
-
保留指数:1118
计算性质
-
辛醇/水分配系数(LogP):0.8
-
重原子数:9
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.142
-
拓扑面积:38
-
氢给体数:2
-
氢受体数:2
安全信息
-
安全说明:S26
-
危险类别码:R36
-
海关编码:2928000090
-
储存条件:应将物品存放在通风、低温和干燥的地方,并与其他库房内的食品原料分开存放。
SDS
SECTION 1: Identification of the substance/mixture and of the company/undertaking
Product identifiers
Product name : Benzylhydrazine
REACH No. : A registration number is not available for this substance as the substance
or its uses are exempted from registration, the annual tonnage does not
require a registration or the registration is envisaged for a later
registration deadline.
Relevant identified uses of the substance or mixture and uses advised against
Identified uses : Laboratory chemicals, Manufacture of substances
SECTION 2: Hazards identification
Classification of the substance or mixture
Classification according to Regulation (EC) No 1272/2008
Acute toxicity, Oral (Category 3), H301
Eye irritation (Category 2), H319
For the full text of the H-Statements mentioned in this Section, see Section 16.
Classification according to EU Directives 67/548/EEC or 1999/45/EC
T Toxic R25
R36
For the full text of the R-phrases mentioned in this Section, see Section 16.
Label elements
Labelling according Regulation (EC) No 1272/2008
Pictogram
Signal word Danger
Hazard statement(s)
H301 Toxic if swallowed.
H319 Causes serious eye irritation.
Precautionary statement(s)
P301 + P310 IF SWALLOWED: Immediately call a POISON CENTER or doctor/
physician.
P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove
contact lenses, if present and easy to do. Continue rinsing.
Supplemental Hazard none
Statements
Other hazards
This substance/mixture contains no components considered to be either persistent, bioaccumulative and
toxic (PBT), or very persistent and very bioaccumulative (vPvB) at levels of 0.1% or higher.
SECTION 3: Composition/information on ingredients
Substances
Molecular weight : 122,17 g/mol
Hazardous ingredients according to Regulation (EC) No 1272/2008
Component Classification Concentration
Benzylhydrazine
Acute Tox. 3; Eye Irrit. 2; <= 100 %
H301, H319
Hazardous ingredients according to Directive 1999/45/EC
Component Classification Concentration
Benzylhydrazine
T, R25R36 <= 100 %
For the full text of the H-Statements and R-Phrases mentioned in this Section, see Section 16
SECTION 4: First aid measures
Description of first aid measures
General advice
Consult a physician. Show this safety data sheet to the doctor in attendance.
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician.
In case of skin contact
Wash off with soap and plenty of water. Take victim immediately to hospital. Consult a physician.
In case of eye contact
Rinse thoroughly with plenty of water for at least 15 minutes and consult a physician.
If swallowed
Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician.
Most important symptoms and effects, both acute and delayed
The most important known symptoms and effects are described in the labelling (see section 2.2) and/or in
section 11
Indication of any immediate medical attention and special treatment needed
No data available
SECTION 5: Firefighting measures
Extinguishing media
Suitable extinguishing media
Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.
Special hazards arising from the substance or mixture
No data available
Advice for firefighters
Wear self-contained breathing apparatus for firefighting if necessary.
Further information
No data available
SECTION 6: Accidental release measures
Personal precautions, protective equipment and emergency procedures
Wear respiratory protection. Avoid dust formation. Avoid breathing vapours, mist or gas. Ensure
adequate ventilation. Evacuate personnel to safe areas. Avoid breathing dust.
For personal protection see section 8.
Environmental precautions
Prevent further leakage or spillage if safe to do so. Do not let product enter drains.
Methods and materials for containment and cleaning up
Pick up and arrange disposal without creating dust. Sweep up and shovel. Keep in suitable, closed
containers for disposal.
Reference to other sections
For disposal see section 13.
SECTION 7: Handling and storage
Precautions for safe handling
Avoid contact with skin and eyes. Avoid formation of dust and aerosols.
Provide appropriate exhaust ventilation at places where dust is formed.
For precautions see section 2.2.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place.
Storage class (TRGS 510): Non-combustible, acute toxic Cat.3 / toxic hazardous materials or hazardous
materials causing chronic effects
Specific end use(s)
Apart from the uses mentioned in section 1.2 no other specific uses are stipulated
SECTION 8: Exposure controls/personal protection
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
Avoid contact with skin, eyes and clothing. Wash hands before breaks and immediately after handling
the product.
Personal protective equipment
Eye/face protection
Face shield and safety glasses Use equipment for eye protection tested and approved under
appropriate government standards such as NIOSH (US) or EN 166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and
the standard EN 374 derived from it.
Body Protection
Complete suit protecting against chemicals, The type of protective equipment must be selected
according to the concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
Where risk assessment shows air-purifying respirators are appropriate use a full-face particle
respirator type N99 (US) or type P2 (EN 143) respirator cartridges as a backup to engineering
controls. If the respirator is the sole means of protection, use a full-face supplied air respirator. Use
respirators and components tested and approved under appropriate government standards such
as NIOSH (US) or CEN (EU).
Control of environmental exposure
Prevent further leakage or spillage if safe to do so. Do not let product enter drains.
SECTION 9: Physical and chemical properties
Information on basic physical and chemical properties
a) Appearance Form: solid
b) Odour No data available
c) Odour Threshold No data available
d) pH No data available
e) Melting point/freezing No data available
point
f) Initial boiling point and No data available
boiling range
g) Flash point No data available
h) Evaporation rate No data available
i) Flammability (solid, gas) No data available
j) Upper/lower No data available
flammability or
explosive limits
k) Vapour pressure No data available
l) Vapour density No data available
m) Relative density No data available
n) Water solubility No data available
o) Partition coefficient: n- No data available
octanol/water
p) Auto-ignition No data available
temperature
q) Decomposition No data available
temperature
r) Viscosity No data available
s) Explosive properties No data available
t) Oxidizing properties No data available
Other safety information
No data available
SECTION 10: Stability and reactivity
Reactivity
No data available
Chemical stability
Stable under recommended storage conditions.
Possibility of hazardous reactions
No data available
Conditions to avoid
No data available
Incompatible materials
Strong oxidizing agents
Hazardous decomposition products
Other decomposition products - No data available
In the event of fire: see section 5
SECTION 11: Toxicological information
Information on toxicological effects
Acute toxicity
No data available
Skin corrosion/irritation
No data available
Serious eye damage/eye irritation
No data available
Respiratory or skin sensitisation
No data available
Germ cell mutagenicity
No data available
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
No data available
Specific target organ toxicity - single exposure
No data available
Specific target organ toxicity - repeated exposure
No data available
Aspiration hazard
No data available
Additional Information
RTECS: Not available
To the best of our knowledge, the chemical, physical, and toxicological properties have not been
thoroughly investigated.
To the best of our knowledge, the chemical, physical, and toxicological properties have not been
thoroughly investigated.
SECTION 12: Ecological information
Toxicity
No data available
Persistence and degradability
No data available
Bioaccumulative potential
No data available
Mobility in soil
No data available
Results of PBT and vPvB assessment
This substance/mixture contains no components considered to be either persistent, bioaccumulative and
toxic (PBT), or very persistent and very bioaccumulative (vPvB) at levels of 0.1% or higher.
Other adverse effects
No data available
SECTION 13: Disposal considerations
Waste treatment methods
Product
Offer surplus and non-recyclable solutions to a licensed disposal company. Dissolve or mix the material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber.
Contaminated packaging
Dispose of as unused product.
SECTION 14: Transport information
UN number
ADR/RID: 2811 IMDG: 2811 IATA: 2811
UN proper shipping name
ADR/RID: TOXIC SOLID, ORGANIC, N.O.S. (Benzylhydrazine)
IMDG: TOXIC SOLID, ORGANIC, N.O.S. (Benzylhydrazine)
IATA: Toxic solid, organic, n.o.s. (Benzylhydrazine)
Transport hazard class(es)
ADR/RID: 6.1 IMDG: 6.1 IATA: 6.1
Packaging group
ADR/RID: III IMDG: III IATA: III
Environmental hazards
ADR/RID: no IMDG Marine pollutant: no IATA: no
Special precautions for user
No data available
SECTION 15: Regulatory information
This safety datasheet complies with the requirements of Regulation (EC) No. 1907/2006.
Safety, health and environmental regulations/legislation specific for the substance or mixture
No data available
Chemical Safety Assessment
For this product a chemical safety assessment was not carried out
SECTION 16: Other information
Full text of H-Statements referred to under sections 2 and 3.
Acute Tox. Acute toxicity
Eye Irrit. Eye irritation
H301 Toxic if swallowed.
H319 Causes serious eye irritation.
Full text of R-phrases referred to under sections 2 and 3
T Toxic
R25 Toxic if swallowed.
R36 Irritating to eyes.
Further information
Copyright 2014 Co. LLC. License granted to make unlimited paper copies for internal use
only.
The above information is believed to be correct but does not purport to be all inclusive and shall be
used only as a guide. The information in this document is based on the present state of our knowledge
and is applicable to the product with regard to appropriate safety precautions. It does not represent any
guarantee of the properties of the product. Corporation and its Affiliates shall not be held
liable for any damage resulting from handling or from contact with the above product. See
and/or the reverse side of invoice or packing slip for additional terms and conditions of sale.
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 苄基硝基胺 Benzylnitramin 19091-99-7 C7H8N2O2 152.153 1,1-二苄肼 N,N-dibenzylhydrazine 5802-60-8 C14H16N2 212.294 —— benzaldehyde benzylhydrazone 13211-11-5 C14H14N2 210.279 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1-苄基氨基胍 benzylamino-guanidine 46121-22-6 C8H12N4 164.21 —— 2-(Benzylhydrazono)-propan 62488-79-3 C10H14N2 162.235 1-苄基氨基甲酰肼 1-benzyl semicarbazide 6635-48-9 C8H11N3O 165.195 —— 1-acetyl-2-benzylhydrazine 7151-53-3 C9H12N2O 164.207 1,1-二苄肼 N,N-dibenzylhydrazine 5802-60-8 C14H16N2 212.294 —— 1-Formyl-1-benzyl-hydrazin 16120-71-1 C8H10N2O 150.18 —— α-(2-Benzyl-hydrazino)-essigsaeure 90556-82-4 C9H12N2O2 180.206 —— 1-benzylhydrazonopropanone 109299-37-8 C10H12N2O 176.218 —— Dithiokohlensaeure-S-methylester- 69664-49-9 C9H12N2S2 212.34 —— benzaldehyde benzylhydrazone 13211-11-5 C14H14N2 210.279
反应信息
-
作为反应物:描述:参考文献:名称:由N'-(芳甲基)肼或1-(芳甲基)-2-(芳基亚甲基)肼无金属合成1,3,4-二唑摘要:摘要 报道了通过氧化脱氢从N '-(芳基甲基)酰肼或1-(芳基甲基)-2-(芳基亚甲基)肼有效且通用的无金属合成1,3,4-恶二唑。通过用乙腈中的(二乙酰氧基碘)苯处理N '-(芳基甲基)酰肼或用[[]处理1-(芳基甲基)-2-(芳基亚甲基)肼来制备一系列2,5-二取代的1,3,4-恶二唑。甲基叔丁基醚中的双(三氟乙酰氧基)碘]苯。醛N-酰基hydr和醛嗪最初是作为中间体原位生成的。 报道了通过氧化脱氢从N '-(芳基甲基)酰肼或1-(芳基甲基)-2-(芳基亚甲基)肼有效且通用的无金属合成1,3,4-恶二唑。通过用乙腈中的(二乙酰氧基碘)苯处理N '-(芳基甲基)酰肼或用[[]处理1-(芳基甲基)-2-(芳基亚甲基)肼来制备一系列2,5-二取代的1,3,4-恶二唑。甲基叔丁基醚中的双(三氟乙酰氧基)碘]苯。醛N-酰基hydr和醛嗪最初是作为中间体原位生成的。DOI:10.1055/s-0034-1379974
-
作为产物:描述:苯甲醛腙 在 K12(Ga4(1,5-bis(2,3-dihydroxybenzamido)naphthalene))6 、 potassium phosphate 、 吡啶硼烷 作用下, 以 重水 为溶剂, 反应 22.0h, 以34%的产率得到苄基肼参考文献:名称:由超分子宿主和吡啶-硼烷辅因子催化的化学选择性和位点选择性还原摘要:超分子催化剂模仿酶的机制,在温和和水性条件下实现大的速率加速和精确的选择性。虽然在超分子宿主促进的小分子合成方面取得了重大进展,但这种反应性在复杂生物分子的化学选择性和位点选择性修饰中的应用实际上仍未得到探索。我们在这里报告了一种超分子系统,其中吡啶-硼烷与各种分子(包括烯酮、酮、醛、肟、腙和亚胺)的共包封在碱性水条件下有效减少。在将未受保护的赖氨酸置于宿主介导的还原胺化条件下后,我们观察到了出色的 ε 选择性,表明在不牺牲反应性的情况下,同一分子内的不同客体结合是可能的。受酶系统对复杂生物分子的翻译后修饰的启发,我们随后将这种超分子反应应用于 11 个氨基酸肽链和人胰岛素中单个赖氨酸残基的位点选择性标记。DOI:10.1021/jacs.0c12479
-
作为试剂:描述:5-ethyl-3-methyllumiflavinium perchlorate 在 苄基肼 作用下, 以 氘代乙腈 为溶剂, 反应 72.0h, 以31%的产率得到4a-Benzyl-5-ethyl-3,7,8,10-tetramethyl-5,10-dihydro-4aH-benzo[g]pteridine-2,4-dione参考文献:名称:Flavin Chemical Models for Monoamine Oxidase Inactivation by Cyclopropylamines, .alpha.-Silylamines, and Hydrazines摘要:Models for the inactivation of the monoamine oxidase A and B, two closely related flavoenzymes, by cyclopropylamines, alpha-silylamines, and hydrazines have been investigated in order to gain insight into the possible chemical mechanisms for these processes. The activated (i.e. high reduction potential and electrophilicity) flavin, 3-methyl-5-ethyllumiflavinium perchlorate (5), was employed in this effort along with trans-2-phenylcyclopropylamine (1), a host of monosubstituted hydrazines (13-16), and alpha-(trimethylsilyl)benzylamine (9). Admixture of 5 with 1 (25 degrees C, MeCN) results in instantaneous formation of the stable and completely characterized flavin-amine adducts 6 (K-e = 2 x 10(4)) derived by addition of the amine function in 1 to the 4a-position of 5. Reaction of the 4a-adduct 6 with cyclopropylamine 1 (85 degrees C, MeCN) cleanly (80%) produces the aldimine 7 formed by condensation of the initial product, trans-cinnamaldehyde and amine 5. These results demonstrate that 4a-adducts related to 6 are capable of undergoing cyclopropane ring opening reactions by polar pathways to produce electrophilic alpha,beta-unsaturated carbonyl products. Consequently, ring opening reactions proposed for monoamine oxidase inactivation by primary and perhaps secondary cyclopropylamines can occur by polar routes and, thus, are not uniquely attributable to radical mechanistic pathways. In a similar manner, the flavinium salt 5 undergoes rapid reaction with the alpha-silylamine 9 to produce a stable 4a-adduct 10 (K-e = 7 x 10(4)). Reaction of this adduct with 9 (45 degrees C, MeCN) leads to initial production of N-[(alpha-trimethylsilyl)benzyl]benzaldimine (12) which undergoes desilylation to produce N-benzylbenzaldimine (11) under these conditions. Also, 4a-adduct 10 is rapidly converted to aldimine 11 by reaction with TBAF at 25 degrees C in MeCN. These results show that 4a-adducts, generated from activated flavins and alpha-silylamines, participate in fragmentation processes leading to silylation of nucleophiles and production of carbonyl products. This polar mechanistic pathway models the known inactivation reactions of the MAOs by alpha-silylamines previously attributed to SET (radical) routes. Reaction of flavinium salt 5 with phenyl- or benzylhydrazine results in formation of 4a-phenyl or -benzyl flavin adducts. For example, admixture of 5 and PhNHNH(2) in CH3CN at 25 degrees C provides the characterizable 4a-phenyl and 4a-cyanomethyl flavins, 21 (28%) and 22 (55%), and benzene. Benzylhydrazine reacts similarly with 5 to produce only the 4a-benzyl adduct 23 (89%). information about the mechanism for adduct formation in these reactions has come from studies with the hydrazine analogs, NH(2)NHCO(2)CH(2)Ph (15) and NH(2)OCH(2)Ph (16). These substances react rapidly with 5 in MeCN at 25 degrees C to cleanly produce stable 4a-hydrazine adducts, 17. The results suggest that 4a-alkylation or -arylation reactions of the activated flavin 5 with hydrazines probably occur via the intermediacy of 4a-hydrazine flavin adducts related to 17. Thus, a polar mechanistic model is also consistent with the known inactivation reactions of the MAOs with hydrazines which are also reported to generate 4a-flavin alkylated and arylated MAO derivatives.DOI:10.1021/ja00106a012
文献信息
-
Synthesis of poly-functionalized pyrazoles under Vilsmeier-Haack reaction conditions作者:Aleksandr V. Popov、Valentina A. Kobelevskaya、Ludmila I. Larina、Igor B. RozentsveigDOI:10.24820/ark.5550190.p010.934日期:——Synthesis of 1,3-disubstituted 5-chloro-1H-pyrazole-4-carbaldehydes was achieved by formylation of the corresponding 5-chloro-1H-pyrazoles under Vilsmeier-Haack conditions.
-
[EN] SUBSTITUTED BENZYLAMINE COMPOUNDS, THEIR USE IN MEDICINE, AND IN PARTICULAR THE TREATMENT OF HEPATITIS C VIRUS (HCV) INFECTION<br/>[FR] COMPOSÉS DE BENZYLAMINE SUBSTITUÉS, LEUR UTILISATION EN MÉDECINE, EN PARTICULIER DANS LE TRAITEMENT D'UNE INFECTION PAR LE VIRUS DE L'HÉPATITE C (VHC)申请人:ASTEX THERAPEUTICS LTD公开号:WO2013064538A1公开(公告)日:2013-05-10The invention provides compounds of the formula (I): or a salt, N-oxide or tautomer thereof, wherein A is CH, CF or nitrogen; E is CH, CF or nitrogen; and R0 is hydrogen or C1-2 alkyl; R1a is selected from CONH2; CO2H; an optionally substituted acyclic C1-8 hydrocarbon group; and an optionally substituted monocyclic carbocyclic or heterocyclic group of 3 to 7 ring members, of which 0, 1, 2, 3 or 4 are heteroatom ring members selected from O, N and S; R2 is selected from hydrogen and a group R2a; R2a is selected from an optionally substituted acyclic d-8 hydrocarbon group; an optionally substituted monocyclic carbocyclic or heterocyclic group of 3 to 7 ring members, of which 0, 1 or 2 ring members are heteroatom ring members selected from O, N and S; and an optionally substituted bicyclic heterocyclic group of 9 or 10 ring members, of which 1 or 2 ring members are nitrogen atoms; wherein at least one of R1 and R2 is other than hydrogen; R3 is an optionally substituted 3- to 10-membered monocyclic or bicyclic carbocyclic or heterocyclic ring containing 0, 1, 2 or 3 heteroatom ring members selected from N, O and S; R4a is selected from halogen; cyano; C1-4 alkyl optionally substituted with one or more fluorine atoms; C1-4 alkoxy optionally substituted with one or more fluorine atoms; hydroxy-C1-4 alkyl; and C1-2 alkoxy-C1-4 alkyl; R5 is selected from hydrogen and a substituent R5a; and R5a is selected from C1-2 alkyl optionally substituted with one or more fluorine atoms; C1-3 alkoxy optionally substituted with one or more fluorine atoms; halogen; cyclopropyl; cyano; and amino, The compounds have activity against hepatitis C virus and can be used in the prevention or treatment of hepatitis C viral infections.该发明提供了以下式(I)的化合物,或其盐、N-氧化物或互变异构体,其中A为CH、CF或氮;E为CH、CF或氮;R0为氢或C1-2烷基;R1a选自CONH2;CO2H;一个可选择取代的非环状C1-8碳氢化合物基团;以及一个可选择取代的含有3至7个环成员的单环碳环或杂环基团,其中0、1、2、3或4个是从O、N和S中选择的杂原子环成员;R2选自氢和一个基团R2a;R2a选自一个可选择取代的非环状d-8碳氢化合物基团;一个可选择取代的含有3至7个环成员的单环碳环或杂环基团,其中0、1或2个环成员是从O、N和S中选择的杂原子环成员;以及一个可选择取代的含有9或10个环成员的双环杂环基团,其中1或2个环成员是氮原子;其中R1和R2中至少一个不是氢;R3选自一个可选择取代的含有0、1、2或3个从N、O和S中选择的杂原子环成员的3至10个成员的单环或双环碳环或杂环环;R4a选自卤素;氰基;C1-4烷基,可选择取代一个或多个氟原子;C1-4烷氧基,可选择取代一个或多个氟原子;羟基-C1-4烷基;和C1-2烷氧基-C1-4烷基;R5选自氢和一个取代基R5a;R5a选自C1-2烷基,可选择取代一个或多个氟原子;C1-3烷氧基,可选择取代一个或多个氟原子;卤素;环丙基;氰基;和氨基。这些化合物对丙型肝炎病毒具有活性,并可用于预防或治疗丙型肝炎病毒感染。
-
Synthesis and anticonvulsant activity of 3-amino-4(3<i>H</i>)-quinazolinones作者:M. J. Kornet、T. Varia、W. BeavenDOI:10.1002/jhet.5570200623日期:1983.11Several 3-amino-4(3H)-quinazolinones were prepared from o-aminobenzoylhydrazines and triethyl orthoformate or from isatoic anhydrides, hydrazines and triethyl orthoformate. o-Aminobenzoylhydrazine intermediates were obtained by reaction of isatoic anhydrides with hydrazines. Some of the aminoquinazolinones displayed anticonvulsant activity in mice.
-
Piperidyindoles as serotonin receptor ligands申请人:——公开号:US20030225068A1公开(公告)日:2003-12-04A pharmaceutical compound of the formula (I) in which R 1 and R 2 are each hydrogen or C 1-6 alkyl, R 3 is —SR 10 , —SOR 10 , —SO 2 R 10 , —COR 10 , —CH 2 OH or —CONHR 11 , where R 10 is C 1-6 alkyl and R 11 is hydrogen or C 1-6 alkyl, R 4 , R 5 , R 6 and R 7 are each hydrogen or C 1-6 alkyl, provided that at least one of R 4 , R 5 , R 6 and R 7 is C 1-6 alkyl, R 8 and R 9 are each hydrogen, halo, C 1-6 alkyl or cyano, n is 0 or 1 and m is 2 or 3, x is a (a) or (b), and y is (c) or (d), wherein R 12 and R 13 are each hydrogen, C 1- alkyl, cyclopropyl or cyclopropyl-C 1-6 alkyl; and salts thereof. 1
-
[EN] PYRIDINONE- AND PYRIDAZINONE-BASED COMPOUNDS AND MEDICAL USES THEREOF<br/>[FR] COMPOSÉS À BASE DE PYRIDINONE ET DE PYRIDAZINONE ET UTILISATIONS MÉDICALES ASSOCIÉES申请人:HLA TIMOTHY公开号:WO2019173790A1公开(公告)日:2019-09-12The various examples presented herein are directed to compounds of the formula A-L1-Het1-L2-Cy1 or a pharmaceutical acceptable salt, polymorph, prodrug, solvate or clathrate thereof, wherein: A is cycloalkyl, aryl, arylalkyl or heterocyclyl; Het1 is heterocyclyl containing at least two heteroatoms; Cy1 is a heterocyclyl; L1 is a bond, alkyl, alkenyl or alkynyl linker; L2 is an acyl or alkyl linker; and A and Cy1 are different. The compounds are useful in the treatment of fibrotic diseases, abnormal vascular leak and pathological angiogenesis.
表征谱图
-
氢谱1HNMR
-
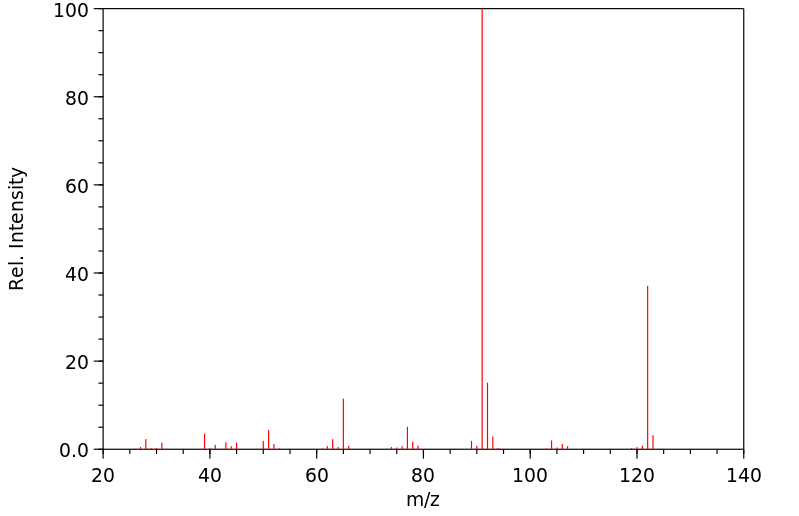
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫