脲基甲酸乙酯 | 626-36-8
中文名称
脲基甲酸乙酯
中文别名
氨基甲酰氨基甲酸乙酯;脲甲酸乙酯;乙基脲基甲酸酯;阿洛酚酸乙酯
英文名称
ethyl allophanate
英文别名
Allophansaeure-ethylester;Allophansaeure-aethylester;ethyl N-carbamoylcarbamate
CAS
626-36-8
化学式
C4H8N2O3
mdl
MFCD00047870
分子量
132.119
InChiKey
PIHPSKJRLDSJPX-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:184-189 °C
-
沸点:190-191℃
-
密度:1.4207 (rough estimate)
-
稳定性/保质期:
远离强氧化剂。
计算性质
-
辛醇/水分配系数(LogP):0.1
-
重原子数:9
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:81.4
-
氢给体数:2
-
氢受体数:3
安全信息
-
危险品标志:Xi
-
安全说明:S24/25
-
危险类别码:R36
-
储存条件:常温常压下保存。
SDS
| Name: | Ethyl Allophanate Material Safety Data Sheet |
| Synonym: | (Aminocarbonyl) Carbamic Acid, Ethyl Este |
| CAS: | 626-36-8 |
Synonym:(Aminocarbonyl) Carbamic Acid, Ethyl Este
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 626-36-8 | Carbamic Acid, (Aminocarbonyl)-, Ethyl | ca 100 | 210-945-2 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation. The toxicological properties of this material have not been fully investigated.
Skin:
May cause skin irritation. The toxicological properties of this material have not been fully investigated.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 626-36-8: Russia: 3 mg/m3 TWA Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: white
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 185 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C4H8N2O3
Molecular Weight: 132.12
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 626-36-8 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Carbamic Acid, (Aminocarbonyl)-, Ethyl Ester - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 626-36-8: No information available.
Canada
CAS# 626-36-8 is listed on Canada's NDSL List.
CAS# 626-36-8 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 626-36-8 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— Diethyl Urea-1,3-dicarboxylate 6620-41-3 C7H12N2O5 204.183 —— N-ethoxycarbonylthiourea 3673-38-9 C4H8N2O2S 148.186 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 N-氨基甲酰氨基甲酸甲酯 allophanic acid methyl ester 761-89-7 C3H6N2O3 118.092 —— diethyl methylenebisallophanate —— C9H16N4O6 276.249
反应信息
-
作为反应物:参考文献:名称:Seibert, Angewandte Chemie, 1959, vol. 71, p. 194摘要:DOI:
-
作为产物:描述:参考文献:名称:Dains; Greider; Kidwell, Journal of the American Chemical Society, 1919, vol. 41, p. 1009摘要:DOI:
文献信息
-
Application of the Guanidine-Acylguanidine Bioisosteric Approach to Argininamide-Type NPY Y2 Receptor Antagonists作者:Nikola Pluym、Albert Brennauer、Max Keller、Ralf Ziemek、Nathalie Pop、Günther Bernhardt、Armin BuschauerDOI:10.1002/cmdc.201100241日期:2011.9.5structural elements, but they compromise the drug‐likeness of numerous biologically active compounds, including ligands of G‐protein‐coupled receptors (GPCRs). As part of a project focused on the search for guanidine bioisosteres, argininamide‐type neuropeptide Y (NPY) Y2 receptor (Y2R) antagonists related to BIIE0246 were synthesized. Starting from ornithine derivatives, NG‐acylated argininamides were obtained诸如胍基等强碱性基团是至关重要的结构元素,但它们会损害许多生物活性化合物的药物样,包括G蛋白偶联受体(GPCR)的配体。作为致力于寻找胍类生物等位基因的项目的一部分,合成了与BIIE0246相关的精氨酸酰胺型神经肽Y(NPY)Y 2受体(Y 2 R)拮抗剂。从鸟氨酸衍生物开始,N ģ -acylated argininamides通过胍基化量身定做的单-Boc保护的得到Ñ -acyl-小号-methylisothioureas。研究了这些化合物的Y 2 R拮抗作用(钙测定),Y 2R亲和力和NPY受体亚型选择性(流式细胞术结合测定)。大多数N G取代的(S)-精氨酰胺显示出与母体化合物相似的Y 2 R拮抗活性和结合亲和力,而带有末端胺的N G酰化或氨基甲酰化类似物则更为出色(Y 2 R:K i和K B值在低纳摩尔范围内)。这表明化合物的碱性虽然比胍的碱度低4-5个数量级,但足以与Y 2的酸性氨基酸形成关键相互作用R
-
Nitration of 2-Substituted Pyrimidine-4,6-diones, Structure and Reactivity of 5,5-<i>gem</i>-Dinitropyrimidine-4,6-diones作者:Abraham Langlet、Nikolaj V. Latypov、Ulf Wellmar、Ulf Bemm、Patrick Goede、Jan Bergman、Ivan RomeroDOI:10.1021/jo025952x日期:2002.11.1in high yields. The gem-dinitro products were easily attacked by nucleophiles with concomitant formation of gem-dinitroacetyl derivatives, which in turn could be further hydrolyzed to salts of dinitromethane and triureas.
-
PHTHALAZINONE DERIVATIVES申请人:Javaid Hashim Muhammad公开号:US20070093489A1公开(公告)日:2007-04-26A compound of the formula (I): wherein: A and B together represent an optionally substituted, fused aromatic ring; D is selected from: (i) where Y 1 is selected from CH and N, Y 2 is selected from CH and N, Y 3 is selected from CH, CF and N; and (ii) and where Q is O or S; R D is: wherein R N1 is selected from H and optionally substituted C 1-10 alkyl; X is selected from a single bond, NR N2 , CR C3 R C4 and C═O; R N2 is selected from H and optionally substituted C 1-10 alkyl; R C3 and R C4 are independently selected from H, R, C(═O)OR, where R is optionally substituted C 1-10 alkyl, optionally substituted C 5-20 aryl or optionally substituted C 3-20 heterocyclyl; Y is selected from NR N3 and CR C1 R C2 ; R C1 and R C2 are independently selected from H, R, C(═O)OR, where R is optionally substituted C 1-10 alkyl, optionally substituted C 5-20 aryl or optionally substituted C 3-20 heterocyclyl; R C1 and R C2 together with the carbon atom to which they are attached may form an optionally substituted spiro-fused C 5-7 carbocylic or heterocyclic ring; and when X is a single bond R N1 and R C2 may together with the N and C atoms to which they are bound, form an optionally substituted C 5-7 heterocylic ring; and when X is CR C3 R C4 , R C2 and R C4 may together form an additional bond, such that there is a double bond between the atoms substituted by R C1 and R C3 .式(I)的化合物:其中:A和B一起代表一个可选择取代的、融合的芳香环;D选择自:(i)其中Y1选择自CH和N,Y2选择自CH和N,Y3选择自CH、CF和N;和(ii)其中Q是O或S;RD是:其中RN1选择自H和可选择取代的C1-10烷基;X选择自单键、NRN2、CRC3RC4和C═O;RN2选择自H和可选择取代的C1-10烷基;RC3和RC4独立选择自H、R、C(═O)OR,其中R是可选择取代的C1-10烷基、可选择取代的C5-20芳基或可选择取代的C3-20杂环烷基;Y选择自NRN3和CRC1RC2;RC1和RC2独立选择自H、R、C(═O)OR,其中R是可选择取代的C1-10烷基、可选择取代的C5-20芳基或可选择取代的C3-20杂环烷基;RC1和RC2与它们连接的碳原子一起可以形成一个可选择取代的螺联的C5-7碳环或杂环;当X是单键时,RN1和RC2可以与它们连接的N和C原子一起形成一个可选择取代的C5-7杂环;当X是CRC3RC4时,RC2和RC4可以一起形成一个额外的键,使得由RC1和RC3取代的原子之间有一个双键。
-
Studies on Organic Sulfur Compounds. XIII. The Oxidation Reaction of Alkoxycarbonylthioureas with Bromine作者:MITSUO NAGANO、MICHIKO OSHIGE、TAKASHI MATSUI、JUNZO TOBITSUKA、KOZO OYAMADADOI:10.1248/cpb.21.2396日期:——N-Alkoxycarbonylthioureas (A) were reacted with bromine in CHCl3 to afford sulfur, alkoxycarbonylureas (B), 3, 5-bis (alkoxycarbonylimino)-1, 2, 4-dithiazolidines (C), 3, 5-bis-(alkoxycarbonylamino)-1, 2, 4-thiadiazoles (D), 2-alkoxycarbonyl-5-alkoxycarbonylamino-3-imino-1, 2, 4-thiadiazolines (E) and 3-amino-5-alkoxycarbonylamino-1, 2, 4-thiadiazoles (F). This paper describes in detail on the confirmation of the structures of these reaction products.
-
Through-Air Drying of Unbacked Tufted Carpets作者:H. Stephen Lee、Wallace W. Carr、Johannes Leisen、Haskell W. BeckhamDOI:10.1177/004051750107100708日期:2001.7
Magnetic resonance imaging (MRI) and a pilot-scale laboratory through-air dryer are used to study through-air drying of unbacked tufted nylon carpets. MRI is used to study the effect of the vacuum extraction procedure on local moisture distribution within the carpet and to measure local moisture profiles during through-air drying where airflow rate is held constant. The effects of several process and carpet construction parameters on drying are discussed.
使用磁共振成像(MRI)和试验规模的透气式干燥机来研究未支撑的绒面尼龙地毯的透气式干燥。 MRI用于研究真空抽提程序对地毯内部局部湿度分布的影响,并在透气式干燥过程中测量局部湿度剖面,其中气流速率保持恒定。讨论了几种工艺和地毯结构参数对干燥的影响。
表征谱图
-
氢谱1HNMR
-
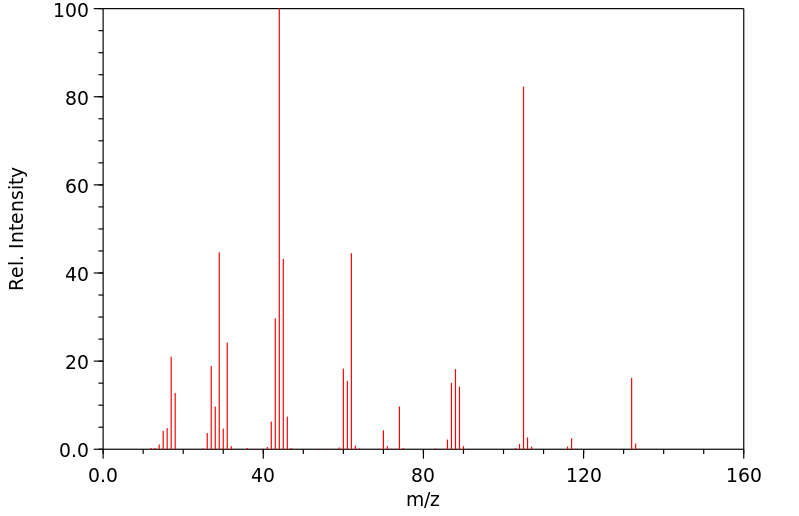
质谱MS
-
碳谱13CNMR
-
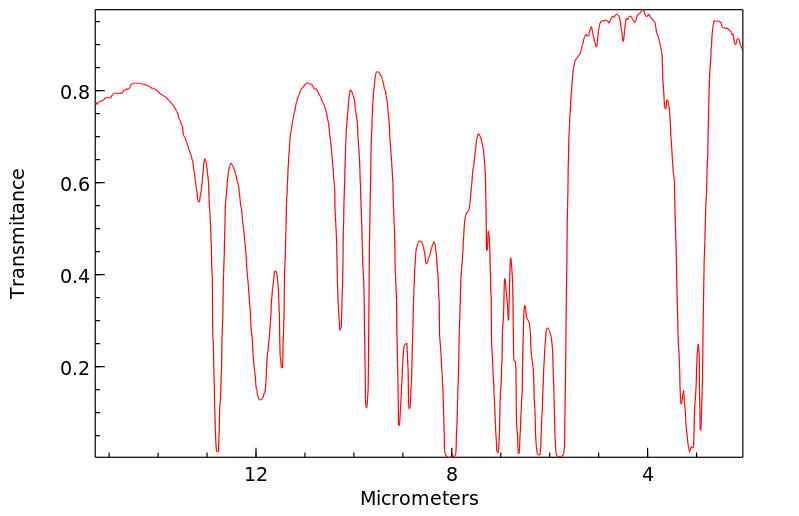
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸