2-氯苯亚甲基丙二腈 | 2698-41-1
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:68-70 °C(lit.)
-
沸点:126 °C0.1 mm Hg(lit.)
-
密度:1.2906 (rough estimate)
-
溶解度:Soluble in acetone, benzene, 1,4-dioxane, ethyl acetate, and methylene chloride (Windholz et al., 1983)
-
暴露限值:NIOSH REL: ceiling 0.05 ppm (0.4 mg/m3), IDLH 2 mg/m3; OSHA PEL: TWA 0.05 ppm; ACGIH TLV: ceiling 0.05 ppm (adopted).
-
物理描述:O-chlorobenzylidene malononitrile appears as white crystalline solid or light beige powder. Odor of pepper. (NTP, 1992)
-
颜色/状态:White crystalline solid
-
气味:Pepper-like odor.
-
蒸汽密度:6.52 (NTP, 1992) (Relative to Air)
-
蒸汽压力:3.4X10-5 mm Hg at 20 °C
-
分解:When heated to decomposition it emits very toxic fumes of /hydrogen chloride, nitrogen oxides and cyanides./
-
保留指数:1516;1555;1516
-
稳定性/保质期:
稳定存储,但不相容于强氧化剂。具有可燃性。
计算性质
-
辛醇/水分配系数(LogP):2.3
-
重原子数:13
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:47.6
-
氢给体数:0
-
氢受体数:2
ADMET
安全信息
-
职业暴露限值:Ceiling: 0.05 ppm (0.4 mg/m3) [skin]
-
危险等级:6.1(a)
-
立即威胁生命和健康浓度:2 mg/m3
-
危险品标志:T
-
安全说明:S26,S27,S36/37/39,S37/39,S45
-
危险类别码:R36/37/38
-
WGK Germany:3
-
危险品运输编号:UN 2647
-
RTECS号:OO3675000
-
海关编码:2926909090
-
包装等级:I
-
危险类别:6.1(a)
-
储存条件:库房应保持低温、通风和干燥,并将物品与食品原料及氧化剂分开存放,实行专人管理。
SDS
Section 1. Identification of the substance
Product Name: [(2-Chlorophenyl)methylene]malononitrile
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: [(2-Chlorophenyl)methylene]malononitrile
CAS number: 2698-41-1
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C10H5ClN2
Molecular weight: 188.6
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides, hydrogen chloride.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
邻氯苄叉缩丙二腈,也称为 CS,是一种美国军队目前使用的防暴剂(RCA)。它于 1928 年合成,并以创造者 Corson 和 Stoughton 的名字命名为“CS”。由于其较高的安全性,CS 在 1959 年取代了氯苯乙酮(CN),成为主要的 RAC。相比 CN,CS 更有效、更安全。它可以通过烟火方式传播,也可以以粉末配方(如 CS1 和 CS2)形式传播。
理化性质邻氯苄叉缩丙二腈是一种可燃的白色结晶固体,具有类似胡椒的气味。它是催泪瓦斯(CS 瓦斯)的主要成分。
用途邻氯苄叉缩丙二腈广泛应用于探索刺激物对人体的影响,作为催泪瓦斯和防暴剂。
危害邻氯苄叉缩丙二腈 (CS) 气雾剂是一种有效的催泪剂,对上呼吸道有刺激作用。CS 暴露的特征效应包括瞬时结膜炎、眼睑痉挛、灼烧感和疼痛。长时间暴露在高浓度下可能会导致肺水肿和严重支气管痉挛。
类别有毒物质
毒性分级高毒
急性毒性- 口服-大鼠 LD50: 178 毫克/公斤
- 口服-小鼠 LD50: 282 毫克/公斤
- 皮肤-兔:12%/6小时 轻度刺激
- 眼睛-兔:1 毫克 轻度刺激
可燃;热分解时排出有毒氮氧化物和氯化物烟雾
储运特性库房低温通风干燥,与食品原料、氧化剂分开存放,专人管理
灭火剂 职业标准- TWA 0.4 毫克/立方米
- CL 0.4 毫克/立方米(皮肤)
上下游信息
反应信息
-
作为反应物:参考文献:名称:6-Amino-5-cyano-1H,4H-pyrazolo[3,4-b]pyrans摘要:DOI:10.1007/bf00506154
-
作为产物:描述:参考文献:名称:一锅顺序反应在中孔碳中金属有机笼的约束自组装摘要:在超分子催化领域中已经广泛探索了定义明确的离散金属有机笼。但是,这些金属有机笼子很少被用作一锅反应的多相催化剂。在这里,我们介绍了氨基官能化介孔碳内含(2,2,6,6-四甲基哌啶-1-基)氧基(TEMPO)的金属有机笼的首次成功的封闭自组装,以实现一个新的双官能团非均相催化剂(Cage @ FDU-ED)进行一锅反应。所获得的双功能催化剂中分离的催化活性位点的正交特征导致增强的催化活性,选择性和可回收性,总转化率可达到96%。这些结果表明,通过精心设计分离的金属有机笼和中孔基质中的催化位点,可以实现连续高效的化学转化。这项研究为金属有机笼子铺平了道路,将其作为异质顺序反应的有前途的平台。DOI:10.1016/j.chempr.2020.06.038
-
作为试剂:描述:参考文献:名称:Franco, M. Luisa T. M. B.; Lazana, M. Celina R. L. R.; Herold, Bernardo J., Journal of the Chemical Society. Perkin transactions II, 1990, # 4, p. 513 - 520摘要:DOI:
文献信息
-
Selective synthesis of E-isomers of aldoximes via a domino aza-Michael/retro-Michael reaction作者:Wei Chen、Wei-Guo Yu、Hai-Bo Shi、Xiao-Yan LuDOI:10.2478/s11696-012-0137-3日期:2012.1.1
Abstract A highly stereoselective synthesis of E-isomer of aldoximes was developed through a base-catalysed domino aza-Michael/retro-Michael reaction of hydroxylamine and 2-(R-benzylidene)malononitrile. This reaction generates (E)-aldoxime diastereomer in high yields (eight examples, isolated yields of 82-93 %), excellent diastereomeric purity (diastereomeric ratio higher than 95: 5 by 1H NMR), and proceeds under mild reaction conditions (aqueous NaOH, pH 12, room temperature, 4 h).
-
Molybdenum carbide as an efficient and durable catalyst for aqueous Knoevenagel condensation作者:Mina Tavakolian、Mohammad Mahdi NajafpourDOI:10.1039/c9nj04647j日期:——and benign catalysts were used for the Knoevenagel condensation reaction. Among the compounds (Mo2C, MoS2, MoB, MoSi2), molybdenum carbide showed efficient performance for the Knoevenagel condensation in aqueous media at room temperature, affording the corresponding products in high yields within a short reaction time. Notably, using this commercially available heterogeneous catalyst, the deaceta
-
Silver Hexafluoroantimonate-Catalyzed Three-Component [2+2+1] Cycloadditions of Allenoates, Dual Activated Olefins, and Isocyanides作者:Jian Li、Yuejin Liu、Chunju Li、Xueshun JiaDOI:10.1002/adsc.201000795日期:2011.4.18The intermolecular [2+2+1] multicomponent cycloadditions from readily available allenoates, dual activated olefins and isocyanides catalyzed by silver hexafluoroantimonate were studied. This protocol allowed the syntheses of highly functionalized five‐membered carbocycles with exclusive regioselectivity and stereoselectivity in an efficient and atom‐economical manner.
-
Structure−Activity Relationship Study of Prion Inhibition by 2-Aminopyridine-3,5-dicarbonitrile-Based Compounds: Parallel Synthesis, Bioactivity, and in Vitro Pharmacokinetics作者:Barnaby C. H. May、Julie A. Zorn、Juanita Witkop、John Sherrill、Andrew C. Wallace、Giuseppe Legname、Stanley B. Prusiner、Fred E. CohenDOI:10.1021/jm061045z日期:2007.1.12-Aminopyridine-3,5-dicarbonitrile compounds were previously identified as mimetics of dominant-negative prion protein mutants and inhibit prion replication in cultured cells. Here, we report findings from a comprehensive structure-activity relationship study of the 6-aminopyridine-3,5-dicarbonitrile scaffold. We identify compounds with significantly improved bioactivity (approximately 40-fold) against
-
Synthesis and characterization of the immobilized Ni–Zn–Fe layered double hydroxide (LDH) on silica-coated magnetite as a mesoporous and magnetically reusable catalyst for the preparation of benzylidenemalononitriles and bisdimedones (tetraketones) under green conditions作者:Masumeh Gilanizadeh、Behzad ZeynizadehDOI:10.1039/c8nj00788h日期:——
Magnetic Fe3O4@SiO2@Ni–Zn–Fe LDH was prepared as an efficient nanocatalyst for the Knoevenagel and tandem Knoevenagel–Michael reactions to afford the products in water.
表征谱图
-
氢谱1HNMR
-
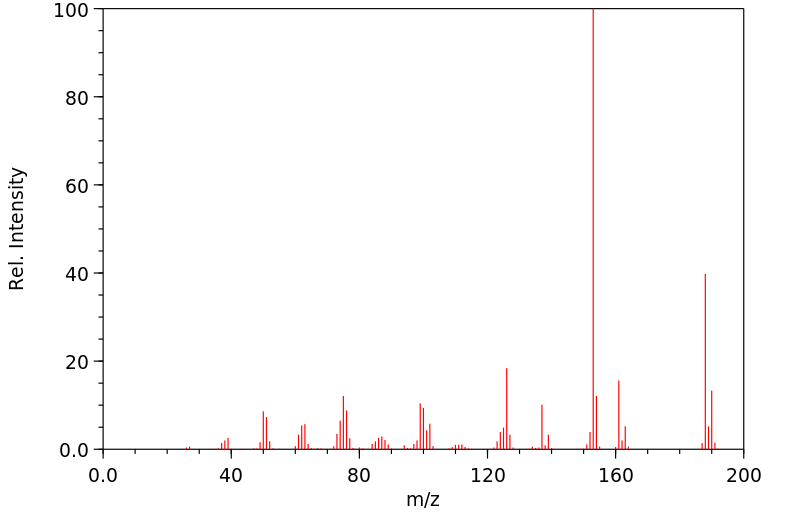
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息