2,5-二羟基苯甲酰胺 | 52405-73-9
中文名称
2,5-二羟基苯甲酰胺
中文别名
——
英文名称
gentisamide
英文别名
2,5-dihydroxybenzamide
CAS
52405-73-9
化学式
C7H7NO3
mdl
——
分子量
153.137
InChiKey
FRXZIFVYTNMCHF-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):1
-
重原子数:11
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:83.6
-
氢给体数:3
-
氢受体数:3
安全信息
-
海关编码:2924299090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 水杨酰胺 salicylamide 65-45-2 C7H7NO2 137.138 2,5-二羟基苯甲酸 2,5-dihydroxybenzoic acid. 490-79-9 C7H6O4 154.122 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 5-(2-aminoethoxy) salicylamide 74454-87-8 C9H12N2O3 196.206 —— 5-[2-(benzylamino)-ethoxy]-salicylamide 76813-69-9 C16H18N2O3 286.331 —— α-[N-[2-(3-carbamoyl-4-hydroxy-phenoxy)-ethyl]-aminomethyl]-benzyl alcohol 89789-86-6 C17H20N2O4 316.357 —— 2-Hydroxy-5-[2-[(2-hydroxy-4-phenylbutyl)amino]ethoxy]benzamide 79407-23-1 C19H24N2O4 344.411 —— 2-Hydroxy-5-[2-[(2-hydroxy-3-phenylpropyl)amino]ethoxy]benzamide 79407-02-6 C18H22N2O4 330.384 —— 2-Hydroxy-5-[2-[(2-hydroxy-6-phenylhexyl)amino]ethoxy]benzamide 89789-89-9 C21H28N2O4 372.464 —— 2-Hydroxy-5-[2-[[2-hydroxy-4-(4-methoxyphenyl)butyl]amino]ethoxy]benzamide 89789-90-2 C20H26N2O5 374.437 —— 1-[2-(3-carbamoyl-4-hydroxyphenoxy)ethylamino]-3-(2-methoxyphenoxy)propan-2-ol 76813-08-6 C19H24N2O6 376.409
反应信息
-
作为反应物:描述:2,5-二羟基苯甲酰胺 在 palladium on activated charcoal 氢气 、 potassium carbonate 、 对甲苯磺酸 作用下, 以 甲醇 、 水 、 异丙醇 为溶剂, 反应 20.0h, 生成 1-[2-(3-carbamoyl-4-hydroxyphenoxy)ethylamino]-3-(2-methoxyphenoxy)propan-2-ol参考文献:名称:.beta.-Adrenergic blocking agents: substituted phenylalkanolamines. Effect of side-chain length on .beta.-blocking potency in vitro摘要:The synthesis of a group of potential beta-blockers bearing a new 5-ethoxysalicylamide substituent on nitrogen is described. These compounds were tested for beta-adrenergic blocking potency in vitro and compared with analogous compounds bearing a tert-butyl group on nitrogen. The new N-substituent increased the beta-blocking potency substantially. In a series of five homologous compounds of the type Ar(CH2)nCHOHCH2NHR (R = 5-ethoxysalicylamide; n = 0-4), two maxima of beta-blocking potency were found for n = 0 and 2. Moreover, the carbon isostere of the corresponding (aryloxy)propanolamine still proved to be a very potent beta-blocker. The ether oxygen in the side chain is therefore not an absolute requirement for activity. Structure-activity relationships are discussed.DOI:10.1021/jm00373a003
-
作为产物:描述:2,5-二羟基苯甲酸 在 ammonium hydroxide 作用下, 反应 6.0h, 以75%的产率得到2,5-二羟基苯甲酰胺参考文献:名称:真菌漆酶催化的核胺化:对羟基苯醌与伯芳香胺的反应产物摘要:Trametes spec。的真菌漆酶(EC 1.10.3.2)催化了对羟基苯醌与伯芳族胺的核胺化反应。和嗜热毁丝霉菌。这是漆酶催化合成氨基醌的首次报道。在氧气存在下将两种化合物与漆酶一起孵育会导致形成相应的单胺化或二价苯醌。没有形成氢醌类化合物。讨论了在不同的对氢醌和芳香胺与不同的漆酶反应过程中观察到的差异。DOI:10.1021/jo048454s
文献信息
-
Derivatives of 3-aminopropane-1,2-diol申请人:Ciba-Geigy Corporation公开号:US04497813A1公开(公告)日:1985-02-05Derivatives of 3-aminopropane-1,2-diol of the formula ##STR1## in which Ar represents optionally substituted aryl, n represents the number 0 or 1, and alk represents alkylene having 2 to 5 carbon atoms, the nitrogen atom and the oxygen atom, or, if n is zero, the phenyl radical, being separated from one another by at least two carbon atoms, and R.sub.1 and R.sub.2, independently of one another, each represents hydrogen or lower alkyl, or together they represent lower alkylene, oxa-lower alkylene, thia-lower alkylene, aza-lower alkylene or N-lower alkyl-aza-lower alkylene, and salts of such compounds, processes for their manufacture, medicaments containing the new compounds and their use for the treatment of Angina pectoris and cardiac arrhythmia, and as blood pressure-reducing agents, as well as for the treatment of reactive or endogenic states of depression.
-
Second Generation Protocol for Multicomponent Coupling Reactions of Aldehydes, Amides and Dienophiles作者:Stefan Klaus、Sandra Hübner、Helfried Neumann、Dirk Strübing、Axel Jacobi von Wangelin、Dirk Gördes、Matthias BellerDOI:10.1002/adsc.200404056日期:2004.7three-component coupling reaction of aldehydes, amides and dienophiles (AAD reaction) has been developed. Compared to known protocols for this type of reaction, the use of aromatic solvents in the presence of dehydrating reagents allows an efficient synthesis of previously not accessible products. Condensation of ubiquitous available aldehydes and amides and subsequent Diels–Alder reactions with dienophiles
-
N-Alkylated aminoalcohols and their pharmaceutical compositions useful申请人:Ciba-Geigy Corporation公开号:US04460580A1公开(公告)日:1984-07-17The invention relates to novel N-alkylated aminoalcohols of the formula ##STR1## in which Ar is a radical of aromatic character which is unsubstituted or substituted by hydroxyl, n has the values nought or 1 and Alk is an alkylene radical having 2 to 5 carbon atoms and the nitrogen atom and the oxygen atom or, if n is nought, the salicylamide radical are separated from one another by at least two carbon atoms in the straight-chain, and their salts. The main action of the novel compounds consists in a stimulation of cardiac .beta.-receptors; the compounds also effect a blockage of adrenergic .alpha.-receptors and a lowering in the blood pressure. They can therefore be used as .beta.-stimulators, especially as agents having a positively inotropic action for the treatment of cardiac insufficiency. Compounds of the formula I in which Ar is phenyl, unsubstituted or substituted by 1 or 2 hydroxyl groups, Alk is an alkylene radical having 2 to 4 carbon atoms, n has the value 1, and the nitrogen atom and the oxygen atom are separated from one another by at least 2 carbon atoms in the straight-chain, or salts thereof, exhibit effects on the central nervous system, which are reflected for example in the suppression of the symptoms of impaired sympathetic functions and in the suppression of lack of initiative. Such compounds of the formula I therefore can be used for the treatment of reactive or endogenic states of depression of varying degrees of severity, and also for the treatment of neurotic or other psychic disturbances involving loss of initiative and depressive disorders. Such compounds can also be used for the short-term treatment of post-partum or postoperative depression, or of depression of different origin. Such compounds of the formula I can be used on their own or in combination with other antidepressants.该发明涉及具有以下结构的新型N-烷基化氨基醇:##STR1##其中Ar是具有芳香性质的基团,未取代或被羟基取代,n的值为零或1,Alk是具有2至5个碳原子的烷基基团,氮原子和氧原子或者,如果n为零,则是水杨酰胺基团,在直链中至少被至少两个碳原子分开,以及它们的盐。这些新型化合物的主要作用在于刺激心脏β-受体;这些化合物还会阻断肾上腺素α-受体并降低血压。因此,它们可以用作β-激动剂,特别是作为具有正性肌力作用的药物,用于治疗心脏功能不全。其中Ar为苯基,未取代或被1或2个羟基取代,Alk是具有2至4个碳原子的烷基基团,n的值为1,氮原子和氧原子在直链中至少被至少2个碳原子分开,或其盐的化合物,对中枢神经系统产生影响,例如抑制受损交感神经功能的症状和抑制缺乏主动性。因此,这些具有结构I的化合物可用于治疗不同程度的反应性或内源性抑郁状态,以及用于治疗神经症或其他涉及缺乏主动性和抑郁障碍的精神紊乱。这些化合物还可用于短期治疗产后抑郁症或术后抑郁症,或不同起源的抑郁症。这些具有结构I的化合物可以单独使用或与其他抗抑郁药物联合使用。
-
COMPOSITIONS AND METHODS FOR TREATMENT OF VIRAL DISEASES申请人:Johansen Lisa M.公开号:US20100009970A1公开(公告)日:2010-01-14The present invention features compositions, methods, and kits useful in the treatment of viral diseases. In certain embodiments, the viral disease is caused by a single stranded RNA virus, a flaviviridae virus, or a hepatic virus. In particular embodiments, the viral disease is viral hepatitis (e.g., hepatitis A, hepatitis B, hepatitis C, hepatitis D, hepatitis E) and the agent or combination of agents includes sertraline, a sertraline analog, UK-416244, or a UK-416244 analog. Also featured are screening methods for identification of novel compounds that may be used to treat a viral disease.
-
Structure-activity relationships of natural quinone vegfrecine analogs with potent activity against VEGFR-1 and -2 tyrosine kinases作者:Hayamitsu Adachi、Chisato Nosaka、Sonoko Atsumi、Koichi Nakae、Yoji Umezawa、Ryuichi Sawa、Yumiko Kubota、Chie Nakane、Masabumi Shibuya、Yoshio NishimuraDOI:10.1038/s41429-021-00445-y日期:2021.10A series of analogs of vegfrecine, a natural quinone vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitor, was synthesized via oxidative amination of 2,5-dihydroxybenzamide with functionalized arylamine followed by ammonolysis and substitution of the quinone ring. The inhibitory activities of the analogs against the VEGFR-1 and -2 tyrosine kinases were assayed in vitro with一系列 vEGfrecine 类似物是一种天然醌血管内皮生长因子受体 (VEGFR) 酪氨酸激酶抑制剂,通过 2,5-二羟基苯甲酰胺与官能化芳胺的氧化胺化,然后氨解和取代醌环合成。体外分析了类似物对 VEGFR-1 和 -2 酪氨酸激酶的抑制活性,目的是确定一种适合治疗癌症和炎症疾病的化合物。检查了苯基官能团的改变、醌环的取代和 1-甲酰胺-2-氨基醌部分的氧化环化以形成异恶唑醌环。在苯环的 5'-位引入卤素和烷基取代基导致对 VEGFR-1 和 -2 酪氨酸激酶的有效抑制。特别是,苯环上 C-5' 处的结构修饰显示出显着影响 VEGFR-1 和 -2 酪氨酸激酶之间抑制的选择性。化合物如图 8 所示,5'-甲基-vEGfrecine 对 VEGFR-2 酪氨酸激酶显示出优于 VEGFR-1 酪氨酸激酶的选择性。
表征谱图
-
氢谱1HNMR
-
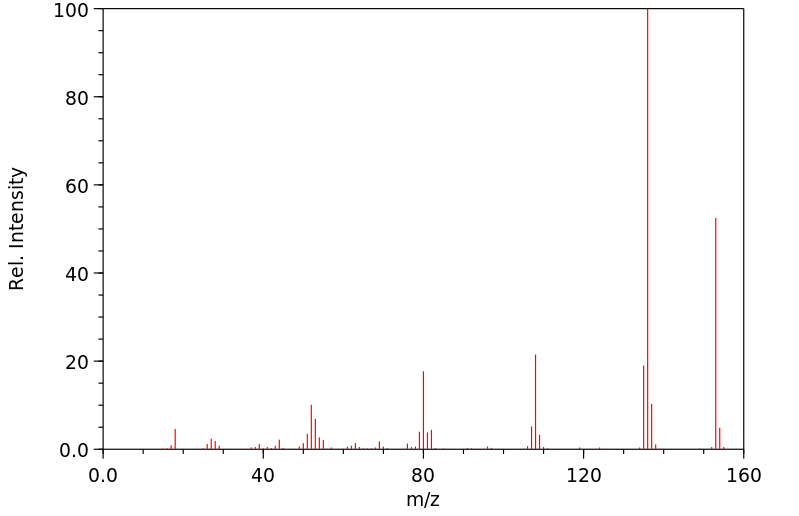
质谱MS
-
碳谱13CNMR
-
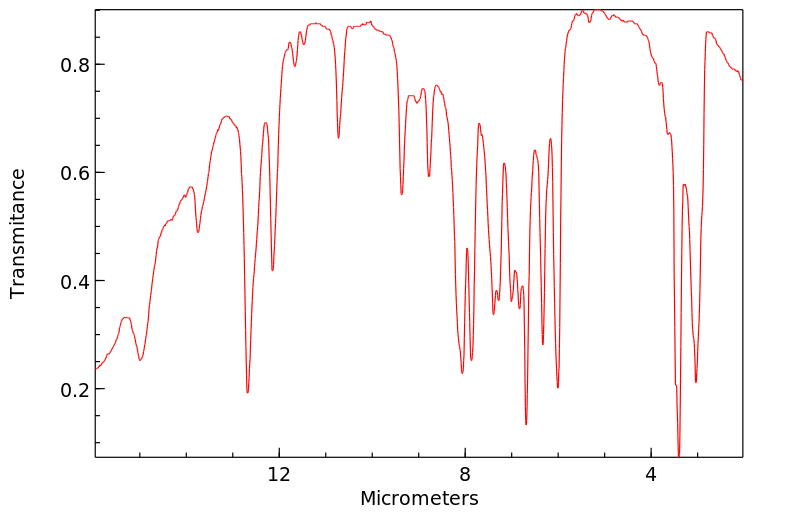
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫