4-氨基吡啶 | 504-24-5
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:155-158 °C (lit.)
-
沸点:273 °C (lit.)
-
密度:1.26
-
闪点:156°C
-
溶解度:在水中的溶解度为50mg/mL,透明,无色
-
LogP:-0.76 at pH7.4
-
物理描述:Pyridine, 4-amino- is a white crystalline material with no odor. Used as an avicide, an intermediate and as a fixer for some textile dyes. (EPA, 1998) It has been approved by the FDA for use as a treatment for multiple sclerosis.
-
颜色/状态:White crystals
-
气味:Odorless
-
蒸汽压力:2.09X10-4 mm Hg at 20 °C
-
稳定性/保质期:
Stable under recommended storage conditions.
-
分解:When heated to decomposition it emits toxic fumes of /nitrogen oxides/.
-
解离常数:pKa = 9.17
-
碰撞截面:117.8 Ų [M+H]+ [CCS Type: TW, Method: calibrated with polyalanine and drug standards]
-
保留指数:1170
计算性质
-
辛醇/水分配系数(LogP):0.3
-
重原子数:7
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:38.9
-
氢给体数:1
-
氢受体数:2
ADMET
安全信息
-
TSCA:Yes
-
危险等级:6.1
-
危险品标志:T+
-
安全说明:S1,S26,S28A,S36/37/39,S37/39,S45,S60,S61
-
危险类别码:R36/37/38,R28
-
WGK Germany:3
-
海关编码:2933399090
-
危险品运输编号:UN 2671 6.1/PG 2
-
危险类别:6.1
-
RTECS号:US1750000
-
包装等级:II
-
危险标志:GHS06
-
危险性描述:H300,H315,H319,H335
-
危险性防范说明:P261,P264,P301 + P310,P305 + P351 + P338
SDS
制备方法与用途
从苯中析出的4-氨基吡啶为无色针状结晶,带有氨气味。它可用作农药、医药及染料中间体,也可作为化学试剂。
合成方法微波辅助脱保护反应将10 mmol基质、10 mmol甲酸铵和2 mmol锌粉悬浮于15 mL乙二醇中,在锥形烧瓶中进行。用160 W微波辐射加热,同时在锥形烧瓶上放置过滤漏斗以防溢出。维持“散热器”以控制输入反应混合物中的微波能量。冷却后,将反应混合物过滤,并用水稀释滤液,然后用乙醚或乙酸乙酯萃取有机层两次,再使用饱和盐水溶液和水洗涤。干燥有机层并过滤蒸发,通过制备TLC或柱色谱纯化得到4-氨基吡啶,产率为95%,熔点158-159℃。
生物活性4-氨基吡啶(4-AP、Fampridine、Dalfampridine)是一种有效的非选择性电压门控钾通道(Kv)抑制剂,在CHO细胞中对Kv1.1和Kv1.2的IC50分别为170 μM和230 μM。
靶点| Target | Value |
|---|---|
| Kv1.4 | 13 μM |
| Kv3.1 | 29 μM |
| Kv3.2 | 100 μM |
| Kv1.3 | 195 μM |
| Kv1.1 | 290 μM |
4-氨基吡啶为无色针状晶体,熔点158-159℃,沸点273℃(180℃时沸点为1.73kPa)。它溶于水和乙醇,略溶于乙醚和苯,难溶于轻油和石油类。
用途用于有机合成,并可作为医药中间体。此外,4-氨基吡啶还用作合成抗生素类药物的中间体。
生产方法将1 g N-氧化-4-硝基吡啶(熔点159℃)溶于30 mL乙酸中,加入2.2 g铁粉反应,保温1 h。冷却后,用水稀释并用30%氢氧化钠溶液调整为强碱性,再用乙醚提取三次,每次100 mL。合并乙醚提取液,并使用无水碳酸钾干燥。蒸发乙醚并减压蒸馏获得4-氨基吡啶粗品。将该粗品从苯或甲苯-石油醚混合溶剂中重结晶可得0.6 g白色针状结晶的4-氨基吡啶,产率为90%。
类别农药
毒性分级剧毒
急性毒性口服 - 大鼠 LD50: 21 毫克/公斤;小鼠 LD50: 42 毫克/公斤
可燃性危险特性明火可燃,高热时释放有毒氮氧化物气体。
储运特性库房应通风、低温干燥。与氧化剂、酸类和食品添加剂分开存放。
灭火剂干粉、泡沫、二氧化碳、砂土
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-氨基吡啶 1-氧化物 1-hydroxypyridin-4-imine 3535-75-9 C5H6N2O 110.115 —— 4-azidopyridine 39910-67-3 C5H4N4 120.114 吡啶 pyridine 110-86-1 C5H5N 79.1014 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4-甲氨基吡啶 4-(Methylamino)pyridine 1121-58-0 C6H8N2 108.143 4-氨基吡啶 1-氧化物 1-hydroxypyridin-4-imine 3535-75-9 C5H6N2O 110.115 4-二甲氨基吡啶 dmap 1122-58-3 C7H10N2 122.17 —— 4-azidopyridine 39910-67-3 C5H4N4 120.114 吡啶 pyridine 110-86-1 C5H5N 79.1014 N-4-吡啶基甲酰胺 N-(pyridin-4-yl)formamide 22236-91-5 C6H6N2O 122.126 4-异氰酸酯吡啶 4-pyridyl isocyanate 70067-45-7 C6H4N2O 120.111 4-(亚磺酰氨基)吡啶 4-(N-sulphinylamino)pyridine 110526-08-4 C5H4N2OS 140.166 N-乙基-4-吡啶胺 4-(ethylamino)pyridine 35036-85-2 C7H10N2 122.17 4-异硫代氰酰基吡啶(9ci) 4-isothiocyanatopyridine 76105-84-5 C6H4N2S 136.177 3,4-二氨基吡啶 pyridine-3,4-diamine 54-96-6 C5H7N3 109.131 4,4,-二吡啶胺 4,4'-dipyridylamine 1915-42-0 C10H9N3 171.202 - 1
- 2
反应信息
-
作为反应物:参考文献:名称:沙梅尔反应从间歇到连续过程的高效换位摘要:研究了桑德迈尔氯化法从批次到安全连续流过程的转换。我们最初的方法是开发一种使用流化学的级联方法,该方法涉及重氮盐的生成并用氯化铜淬灭。为了实现这种安全的连续过程重氮化,使用了化学计量方法(Simplex方法)并外推以建立完全连续流方法。还通过合成几种(杂)芳基氯来检查反应范围。还对过程进行了验证和放大。在提高安全性的同时获得了更高的生产率。DOI:10.1021/acs.oprd.6b00318
-
作为产物:描述:4-氰基吡啶 以90.8的产率得到4-氨基吡啶参考文献:名称:ONE-POT PROCESS FOR THE SYNTHESIS OF DALFAMPRIDINE摘要:一种从4-吡啶腈出发,采用一锅法制备Dalfampridine(1),即4-氨基吡啶的方法。该方法无需分离中间体,在环境、收率、生产率和产物纯度方面特别有优势,无论是在反应混合物中还是在分离的晶体中。公开号:US20110319628A1
-
作为试剂:描述:水 、 乙二胺 、 copper(II) chloride dihydrate 、 ethylenediphosphonic acid 、 在 4-氨基吡啶 、 lithium tetraborate 、 europium(III) nitrate hexahydrate 作用下, 生成参考文献:名称:含有机二磷酸酯的多铌酸环及其通过氢键组装成三维骨架摘要:在这项工作中,一种新型的含有机二磷酸盐的无机-有机杂化聚氧铌酸盐(PONb)环{(PO 3 CH 2 CH 2 PO 3 H) 4 Nb 8 O 16 } 4– ( Nb 8 P 8 )已通过一个方法实现-锅式水热法。该环由四方{Nb 8 O 36 }基序和四个{PO 3 CH 2 CH 2 PO 3 H}配体构成。有趣的是, Nb 8 P 8可以通过K–H 2 O簇{K 2 (H 2 O) 4 (OH) 2 }连接在一起形成一维链{[K 2 (H 2 O) 4 (OH) 2 ] Nb 8 P 8 } n并进一步通过 {Cu(en) 2 } 2+ (en = 乙二胺) 配合物连接,形成三维超分子框架 {[Cu(en) 2 ] 2 [K 2 (H 2 O) 4 (OH) 2 ] Nb 8 P 8 }·3en·H 2 O ( 1 )。 1表现出良好的化学和热稳定性,在298 K时具有≤224 cmDOI:10.1021/acs.inorgchem.4c00741
文献信息
-
<i>N</i>-Ammonium Ylide Mediators for Electrochemical C–H Oxidation作者:Masato Saito、Yu Kawamata、Michael Meanwell、Rafael Navratil、Debora Chiodi、Ethan Carlson、Pengfei Hu、Longrui Chen、Sagar Udyavara、Cian Kingston、Mayank Tanwar、Sameer Tyagi、Bruce P. McKillican、Moses G. Gichinga、Michael A. Schmidt、Martin D. Eastgate、Massimiliano Lamberto、Chi He、Tianhua Tang、Christian A. Malapit、Matthew S. Sigman、Shelley D. Minteer、Matthew Neurock、Phil S. BaranDOI:10.1021/jacs.1c03780日期:2021.5.26taking a first-principles approach guided by computation, these new mediators were identified and rapidly expanded into a library using ubiquitous building blocks and trivial synthesis techniques. The ylide-based approach to C–H oxidation exhibits tunable selectivity that is often exclusive to this class of oxidants and can be applied to real-world problems in the agricultural and pharmaceutical sectors强 C(sp 3 )-H 键的位点特异性氧化在有机合成中具有无可争议的效用。从简化对代谢物的获取和先导化合物的后期多样化到截断逆合成计划,学术界和工业界都越来越需要新的试剂和方法来实现这种转变。当前化学试剂的一个主要缺点是在结构和反应性方面缺乏多样性,这阻碍了用于快速筛选的组合方法的使用。在这方面,定向进化仍然最有希望在各种复杂环境中实现复杂的 C-H 氧化。在此,我们提出了一个设计合理的平台,该平台使用N-铵叶立德作为电化学驱动的氧化剂,用于位点特异性、化学选择性 C(sp 3 )-H 氧化。通过采用以计算为指导的第一性原理方法,这些新的介质被识别出来,并使用无处不在的构建块和简单的合成技术迅速扩展到一个库中。基于叶立德的 C-H 氧化方法表现出可调的选择性,这通常是此类氧化剂独有的,可应用于农业和制药领域的实际问题。
-
[EN] THIOPHENE DERIVATIVES FOR THE TREATMENT OF DISORDERS CAUSED BY IGE<br/>[FR] DÉRIVÉS DE THIOPHÈNE POUR LE TRAITEMENT DE TROUBLES PROVOQUÉS PAR IGE申请人:UCB BIOPHARMA SRL公开号:WO2019243550A1公开(公告)日:2019-12-26Thiophene derivatives of formula (I) and a pharmaceutically acceptable salt thereof are provided. These compounds have utility for the treatment or prevention of disorders caused by IgE, such as allergy, type 1 hypersensitivity or familiar sinus inflammation.
-
[EN] SUBSTITUTED BENZYLAMINE COMPOUNDS, THEIR USE IN MEDICINE, AND IN PARTICULAR THE TREATMENT OF HEPATITIS C VIRUS (HCV) INFECTION<br/>[FR] COMPOSÉS DE BENZYLAMINE SUBSTITUÉS, LEUR UTILISATION EN MÉDECINE, EN PARTICULIER DANS LE TRAITEMENT D'UNE INFECTION PAR LE VIRUS DE L'HÉPATITE C (VHC)申请人:ASTEX THERAPEUTICS LTD公开号:WO2013064538A1公开(公告)日:2013-05-10The invention provides compounds of the formula (I): or a salt, N-oxide or tautomer thereof, wherein A is CH, CF or nitrogen; E is CH, CF or nitrogen; and R0 is hydrogen or C1-2 alkyl; R1a is selected from CONH2; CO2H; an optionally substituted acyclic C1-8 hydrocarbon group; and an optionally substituted monocyclic carbocyclic or heterocyclic group of 3 to 7 ring members, of which 0, 1, 2, 3 or 4 are heteroatom ring members selected from O, N and S; R2 is selected from hydrogen and a group R2a; R2a is selected from an optionally substituted acyclic d-8 hydrocarbon group; an optionally substituted monocyclic carbocyclic or heterocyclic group of 3 to 7 ring members, of which 0, 1 or 2 ring members are heteroatom ring members selected from O, N and S; and an optionally substituted bicyclic heterocyclic group of 9 or 10 ring members, of which 1 or 2 ring members are nitrogen atoms; wherein at least one of R1 and R2 is other than hydrogen; R3 is an optionally substituted 3- to 10-membered monocyclic or bicyclic carbocyclic or heterocyclic ring containing 0, 1, 2 or 3 heteroatom ring members selected from N, O and S; R4a is selected from halogen; cyano; C1-4 alkyl optionally substituted with one or more fluorine atoms; C1-4 alkoxy optionally substituted with one or more fluorine atoms; hydroxy-C1-4 alkyl; and C1-2 alkoxy-C1-4 alkyl; R5 is selected from hydrogen and a substituent R5a; and R5a is selected from C1-2 alkyl optionally substituted with one or more fluorine atoms; C1-3 alkoxy optionally substituted with one or more fluorine atoms; halogen; cyclopropyl; cyano; and amino, The compounds have activity against hepatitis C virus and can be used in the prevention or treatment of hepatitis C viral infections.该发明提供了以下式(I)的化合物,或其盐、N-氧化物或互变异构体,其中A为CH、CF或氮;E为CH、CF或氮;R0为氢或C1-2烷基;R1a选自CONH2;CO2H;一个可选择取代的非环状C1-8碳氢化合物基团;以及一个可选择取代的含有3至7个环成员的单环碳环或杂环基团,其中0、1、2、3或4个是从O、N和S中选择的杂原子环成员;R2选自氢和一个基团R2a;R2a选自一个可选择取代的非环状d-8碳氢化合物基团;一个可选择取代的含有3至7个环成员的单环碳环或杂环基团,其中0、1或2个环成员是从O、N和S中选择的杂原子环成员;以及一个可选择取代的含有9或10个环成员的双环杂环基团,其中1或2个环成员是氮原子;其中R1和R2中至少一个不是氢;R3选自一个可选择取代的含有0、1、2或3个从N、O和S中选择的杂原子环成员的3至10个成员的单环或双环碳环或杂环环;R4a选自卤素;氰基;C1-4烷基,可选择取代一个或多个氟原子;C1-4烷氧基,可选择取代一个或多个氟原子;羟基-C1-4烷基;和C1-2烷氧基-C1-4烷基;R5选自氢和一个取代基R5a;R5a选自C1-2烷基,可选择取代一个或多个氟原子;C1-3烷氧基,可选择取代一个或多个氟原子;卤素;环丙基;氰基;和氨基。这些化合物对丙型肝炎病毒具有活性,并可用于预防或治疗丙型肝炎病毒感染。
-
Enzyme-Responsive Silica Mesoporous Supports Capped with Azopyridinium Salts for Controlled Delivery Applications作者:Núria Mas、Alessandro Agostini、Laura Mondragón、Andrea Bernardos、Félix Sancenón、M. Dolores Marcos、Ramón Martínez-Máñez、Ana M. Costero、Salvador Gil、Matilde Merino-Sanjuán、Pedro Amorós、Mar Orzáez、Enrique Pérez-PayáDOI:10.1002/chem.201202740日期:2013.1.21The preparation of a new capped silica mesoporous material, Rh‐Azo‐S, for on‐command delivery applications in the presence of target enzymes is described. The material consists of nanometric mesoporous MCM‐41‐like supports loaded with Rhodamine B and capped with an azopyridine derivative. The material was designed to show “zero delivery” and to display a cargo release in the presence of reductases描述了一种新的加盖的二氧化硅介孔材料Rh-Azo-S的制备,用于在目标酶存在下按需递送的应用。该材料由纳米级中孔MCM-41-样载体组成,载体中装有若丹明B,并用偶氮吡啶衍生物封端。该材料设计为显示“零传递”并在存在还原酶和酯酶的情况下显示货物释放,还原酶和酯酶通常存在于结肠中,这主要是由于肠道菌群引起的。评估了Rh-Azo-S体外研究的开放性和货物释放性,发现它们是在存在这些酶的情况下发生的,而在存在胃蛋白酶的情况下则没有观察到传递。此外,Rh‐Azo‐S纳米颗粒被用来研究若丹明B染料在细胞内介质中的受控递送。HeLa细胞用于测试纳米颗粒的“非”毒性。此外,通过共聚焦显微镜证实了染料通过内部化和酶介导的门打开在这些细胞中的传递。此外,还制备了载有Azo基团并装载了细胞毒性喜树碱(CPT)的纳米颗粒(固体CPT‐Azo‐S),并用作HeLa细胞中的递送纳米装置。当使用这种固体时,由于纳米粒
-
[EN] SUBSTITUTED PYRIDAZINE CARBOXAMIDE COMPOUNDS AS KINASE INHIBITOR COMPOUNDS<br/>[FR] COMPOSÉS DE PYRIDAZINECARBOXAMIDE SUBSTITUÉS UTILES EN TANT QUE COMPOSÉS INHIBITEURS DE KINASE
表征谱图
-
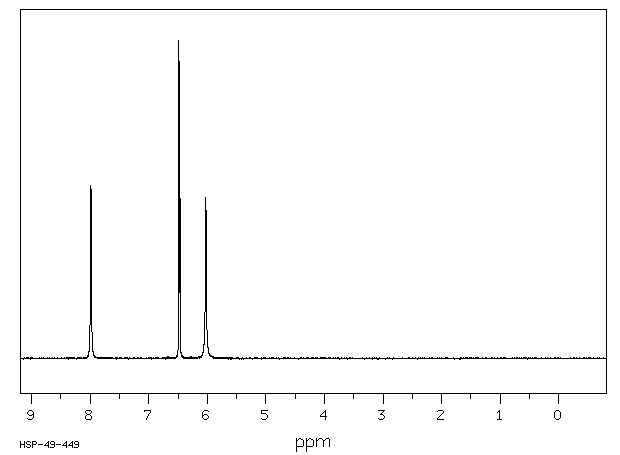
氢谱1HNMR
-
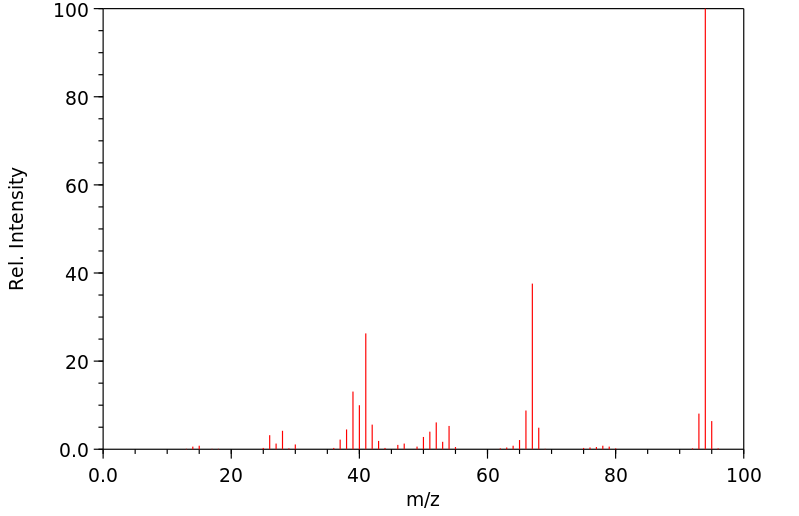
质谱MS
-
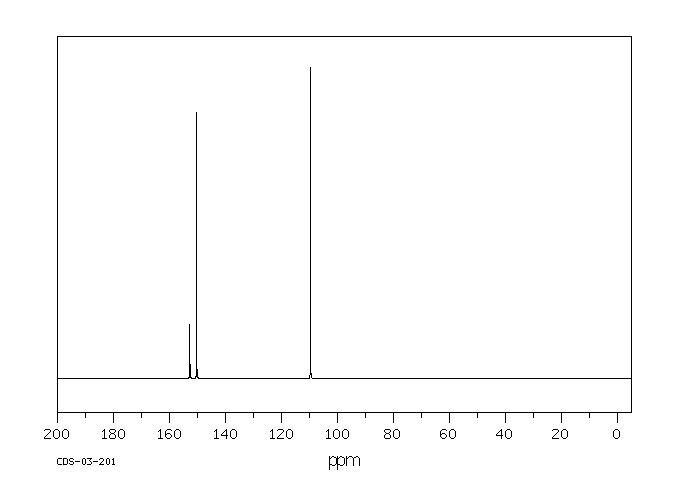
碳谱13CNMR
-
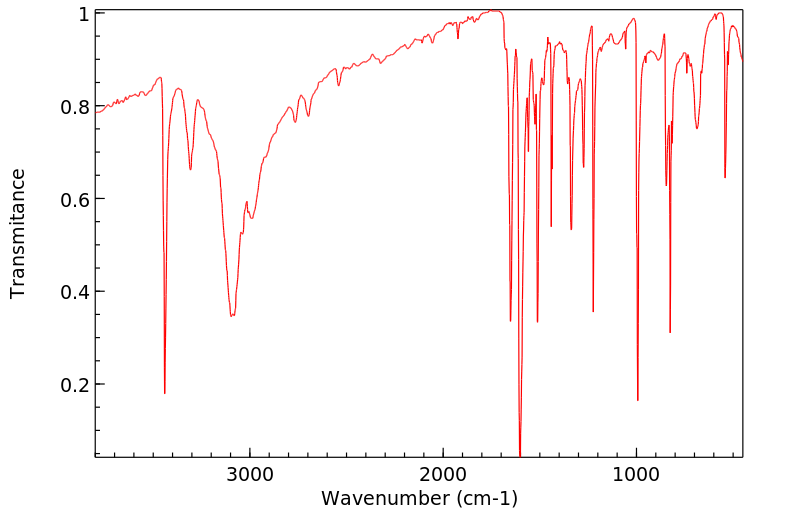
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息