间甲苯甲酸酰肼 | 13050-47-0
中文名称
间甲苯甲酸酰肼
中文别名
3-甲基苯甲酰肼;间苯甲酰肼;间甲苯甲酰肼
英文名称
3-methylbenzoyl hydrazine
英文别名
3-methylbenzohydrazide;3-methylbenzoic acid hydrazide
CAS
13050-47-0
化学式
C8H10N2O
mdl
MFCD00014760
分子量
150.18
InChiKey
XFNNAMBYJSQXKF-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:96 °C
-
稳定性/保质期:
在常温常压下稳定,避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):0.7
-
重原子数:11
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.125
-
拓扑面积:55.1
-
氢给体数:2
-
氢受体数:2
安全信息
-
危险等级:IRRITANT
-
安全说明:S26,S36
-
危险类别码:R36/37/38
-
海关编码:2928000090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:请将物品存放在常温、密闭、避光、通风和干燥的地方。
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
M-Toluic acid hydrazide
Product Name:
Synonyms: 3-Methylbenzohydrazide
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H315: Causes skin irritation
H319: Causes serious eye irritation
H335: May cause respiratory irritation
P261: Avoid breathing dust/fume/gas/mist/vapours/spray
Wear protective gloves/protective clothing/eye protection/face protection
P280:
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
P304+P340: IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing
P405: Store locked up
Section 3. Composition/information on ingredients.
M-Toluic acid hydrazide
Ingredient name:
CAS number: 13050-47-0
Section 4. First aid measures
Immediately wash skin with copious amounts of water for at least 15 minutes while removing
Skin contact:
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Ingestion:
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Not specified
Appearance:
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C8H10N2O
Molecular weight: 150.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
M-Toluic acid hydrazide
Product Name:
Synonyms: 3-Methylbenzohydrazide
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H315: Causes skin irritation
H319: Causes serious eye irritation
H335: May cause respiratory irritation
P261: Avoid breathing dust/fume/gas/mist/vapours/spray
Wear protective gloves/protective clothing/eye protection/face protection
P280:
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
P304+P340: IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing
P405: Store locked up
Section 3. Composition/information on ingredients.
M-Toluic acid hydrazide
Ingredient name:
CAS number: 13050-47-0
Section 4. First aid measures
Immediately wash skin with copious amounts of water for at least 15 minutes while removing
Skin contact:
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Ingestion:
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Not specified
Appearance:
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C8H10N2O
Molecular weight: 150.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1,2-di-m-toluoylhydrazine 59646-36-5 C16H16N2O2 268.315 间甲苯乙酰胺 m-toluamide 618-47-3 C8H9NO 135.166 —— N'-isopropyl-3-methylbenzohydrazide 15563-22-1 C11H16N2O 192.261 —— 3-methyl-N-(propan-2-ylideneamino)benzamide —— C11H14N2O 190.245 —— [(3-Methylbenzoyl)amino]urea 1256844-24-2 C9H11N3O2 193.205 —— 1-(m-toluoyl)-3-thiosemicarbazide 881-73-2 C9H11N3OS 209.272 —— 3-methyl-benzoic acid benzylidenehydrazide 77420-61-2 C15H14N2O 238.289 —— N-(benzylideneamino)-3-methylbenzamide 77420-61-2 C15H14N2O 238.289 —— PCM-0102737 862853-27-8 C10H11ClN2O2 226.663 —— (E)-3-methyl-N'-(3-methylbenzylidene)benzohydrazide —— C16H16N2O 252.316 —— 3-<2-(3-methylbenzoyl)hydrazino>-1-propanesulphonic acid 106710-48-9 C11H16N2O4S 272.325 —— N-[(4-hydroxyphenyl)methylideneamino]-3-methylbenzamide 247085-84-3 C15H14N2O2 254.288 3-甲基苯甲酰叠氮化物 3-methylbenzoyl azide 71313-13-8 C8H7N3O 161.163 —— N'-(butoxycarbonylethyl)-3-methylbenzohydrazide 1156004-53-3 C15H22N2O3 278.351 —— (Z)-4-[N'-(3-Methyl-benzoyl)-hydrazino]-4-oxo-but-2-enoic acid —— C12H12N2O4 248.238 —— N-[(2-chlorophenyl)methylideneamino]-3-methylbenzamide 324055-11-0 C15H13ClN2O 272.734 —— N′-(2-hydroxybenzylidene)-3-methylbenzohydrazide 82859-73-2 C15H14N2O2 254.288 —— 3-methyl-N'-(pyridin-2-ylmethylene)benzohydrazide —— C14H13N3O 239.277 —— N-(m-methylbenzamido)-N'-phenylthiourea 135841-63-3 C15H15N3OS 285.37 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:Mohan, Jag; Anjaneyulu, G. S. R.; Kiran, Indian Journal of Chemistry - Section B Organic and Medicinal Chemistry, 1988, vol. 27, # 1-12, p. 128 - 131摘要:DOI:
-
作为产物:参考文献:名称:三种新的芳酰hydr配体的氧化钒(V)配合物的合成,晶体结构和类胰岛素活性。摘要:通过C,H,N元素分析,单晶X射线衍射,UV / Vis和IR光谱设计,合成和表征了三种新型的氧化钒(V)配合物。配合物1:[VOL 1 X](H 2 L 1 =(E)-N'-(2-羟基亚苄基)-3-甲基苯并肼,HX =乙基麦芽酚= 2-乙基-3-羟基-4-吡喃酮),配合物2 : [VOL 2(CH 3 O)(CH 3 OH)],(H 2 L 2 = C 16 H 16 N 2 O 4 =(E)-N'-(2-羟基苄叉)-3,5-二甲氧基苯甲酰肼,通道3OH =甲醇),络合物3:[VOL 3 X](H 2 L 3 =(E)-N′-(3-乙氧基-2-羟基亚苄基)-3,5-二甲氧基苯并肼)。测试了三种复合物的胰岛素样活性。正常和链脲佐菌素(STZ)糖尿病小鼠均在胃内给药两周。发现以10.0和5.0mg V·kg -1的剂量的复合物可以显着降低STZ糖尿病小鼠的血糖水平,并且治疗的正常小鼠DOI:10.1016/j.jinorgbio.2019.03.020
文献信息
-
Design and optimization of N-acylhydrazone pyrimidine derivatives as E. coli PDHc E1 inhibitors: Structure-activity relationship analysis, biological evaluation and molecular docking study作者:Haifeng He、Hongying Xia、Qin Xia、Yanliang Ren、Hongwu HeDOI:10.1016/j.bmc.2017.08.038日期:2017.10binding site of Escherichia coli (E. coli) pyruvate dehydrogenase multienzyme complex E1 (PDHc E1), a series of novel ‘open-chain’ classes of ThDP analogs A, B, and C with N-acylhydrazone moieties was designed and synthesized to explore their activities against E. coli PHDc E1 in vitro and their inhibitory activity against microbial diseases were further evaluated in vivo. As a result, A1–23 exhibited moderate通过靶向硫胺二磷酸(THDP)结合位点的大肠杆菌(大肠杆菌)丙酮酸脱氢酶多酶复合物E1(PDHC E1),一系列的THDP小说“开链”类的类似物甲,乙,和Ç与ñ -设计并合成了酰基to部分,以探讨它们在体外对大肠杆菌PHDc E1的活性,并在体内进一步评价其对微生物疾病的抑制作用。结果,A1 – 23对大肠杆菌PDHc E1表现出了中度到强效的抑制活性(IC 50 = 0.15–23.55μM)。有效的抑制剂A13,A14,A15,C2具有很强的抑制活性,对大肠杆菌PDHc E1的IC 50值为0.60、0.15、0.39和0.34μM,并且在微生物和哺乳动物之间具有良好的酶选择性抑制作用。特别是,最有效的抑制剂A14可以控制99.37%的米地黄单胞菌(Xanthimonas oryzae pv)。Oryzae。此外,化合物A14在大肠杆菌中的结合特征对PDHc E1进行了研究,以通过分子
-
The α-Effect in Hydrazinolysis of 4-Chloro-2-Nitrophenyl X-Substituted-Benzoates: Effect of Substituent X on Reaction Mechanism and the α-Effect作者:Min-Young Kim、Tae-Eun Kim、Jieun Lee、Ik-Hwan UmDOI:10.5012/bkcs.2014.35.8.2271日期:2014.8.20Second-order rate constants (
$k_N$ ) have been measured spectrophotometrically for the reaction of 4-chloro-2-nitrophenyl X-substituted-benzoates (6a-6h) with a series of primary amines including hydrazine in 80 mol %$H_2O$ /20 mol % DMSO at$25.0^\circ}C$ . The Br$\o}$ nsted-type plot for the reaction of 4-chloro-2-nitrophenyl benzoate (6d) is linear with$\beta}_nuc}$ = 0.74 when hydrazine is excluded from the correlation. Such a linear Br$\o}$ nsted-type plot is typical for reactions reported previously to proceed through a stepwise mechanism in which expulsion of the leaving group occurs in the rate-determining step (RDS). The Hammett plots for the reactions of 6a-6h with hydrazine and glycylglycine are nonlinear. In contrast, the Yukawa-Tsuno plots exhibit excellent linear correlations with$\rho}_X$ = 1.29-1.45 and r = 0.53-0.56, indicating that the nonlinear Hammett plots are not due to a change in RDS but are caused by resonance stabilization of the substrates possessing an electron-donating group (EDG). Hydrazine is ca. 47-93 times more reactive than similarly basic glycylglycine toward 6a-6h (e.g., the$\alpha}$ -effect). The$\alpha}$ -effect increases as the substituent X in the benzoyl moiety becomes a stronger electron-withdrawing group (EWG), indicating that destabilization of the ground state (GS) of hydrazine through the repulsion between the nonbonding electron pairs on the two N atoms is not solely responsible for the substituent-dependent$\alpha}$ -effect. Stabilization of transition state (TS) through five-membered cyclic TSs, which would increase the electrophilicity of the reaction center or the nucleofugality of the leaving group, contributes to the$\alpha}$ -effect observed in this study.二次速率常数($k_N$ )已通过分光光度法测定,用于4-氯-2-硝基苯基X取代苯甲酸酯(6a-6h)与一系列伯胺(包括80摩尔%$H_2O$ /20摩尔% DMSO中的25.0°C下的联氨)的反应。当联氨被排除在相关性之外时,4-氯-2-硝基苯基苯甲酸酯(6d)反应的Br$\o}$ nsted型图是线性的,$\beta}_nuc}$ = 0.74。这种线性Br$\o}$ nsted型图是典型的反应,先前报道这些反应通过逐步机制进行,其中离去基团的排出发生在速率决定步骤(RDS)中。6a-6h与联氨和甘氨酰甘氨酸反应的Hammett图是非线性的。相比之下,Yukawa-TSuno图显示出极佳的线性相关性,$\rho}_X$ = 1.29-1.45,r = 0.53-0.56,表明非线性Hammett图并非由于RDS的变化,而是由于具有供电子基团(EDG)的底物的共振稳定化所导致。联氨对6a-6h的反应性大约是同样碱性的甘氨酰甘氨酸的47-93倍(例如,$\alpha}$ 效应)。随着苯甲酰基中取代基X成为更强的吸电子基团(EWG),$\alpha}$ 效应增加,表明通过两个N原子上的非键电子对之间的排斥来破坏联氨的基态(GS)并不是唯一导致取代基依赖的$\alpha}$ 效应的原因。通过五元环过渡态(TS)的稳定化,这将增加反应中心的亲电性或离去基团的离核性,有助于在本研究中观察到的$\alpha}$ 效应。 -
Discovery of Functionally Selective 7,8,9,10-Tetrahydro-7,10-ethano-1,2,4-triazolo[3,4-<i>a</i>]phthalazines as GABA<sub>A</sub> Receptor Agonists at the α<sub>3</sub> Subunit作者:Michael G. N. Russell、Robert W. Carling、John R. Atack、Frances A. Bromidge、Susan M. Cook、Peter Hunt、Catherine Isted、Matt Lucas、Ruth M. McKernan、Andrew Mitchinson、Kevin W. Moore、Robert Narquizian、Alison J. Macaulay、David Thomas、Sally-Anne Thompson、Keith A. Wafford、José L. CastroDOI:10.1021/jm040883v日期:2005.3.1We have previously identified the 7,8,9,10-tetrahydro-7,10-ethano-1,2,4-triazolo[3,4-a]phthalazine (1) as a potent partial agonist for the alpha(3) receptor subtype with 5-fold selectivity in binding affinity over alpha(1). This paper describes a detailed investigation of the substituents on this core structure at both the 3- and 6-positions. Despite evaluating a wide range of groups, the maximum selectivity我们之前已经确定了7,8,9,10-四氢-7,10-乙醇-1,2,4-三唑并[3,4-a]酞嗪(1)是α(3)的有效部分激动剂。受体亚型,对α(1)的结合亲和力具有5倍的选择性。本文描述了在此核心结构的3位和6位上的取代基的详细研究。尽管评估了广泛的组,但相对于alpha(1)亚型,对alpha(3)亚型的亲和力可达到的最大选择性是12倍(对于57)。尽管大多数类似物在功效上均未显示选择性,但一些类似物确实在α(1)处表现出部分激动作用,而在α(3)处表现出拮抗作用(例如25和75)。但是,测试了两个类似物(93和96),它们都在6位上有三唑取代基,显示出对alpha(3)亚型的疗效明显高于alpha(1)亚型。这是该系列中可以在所需方向上实现选择性的第一个迹象。
-
Synthesis, in vitro α-glucosidase inhibitory activity and molecular docking studies of new thiazole derivatives作者:Khalid Mohammed Khan、Saira Qurban、Uzma Salar、Muhammad Taha、Shafqat Hussain、Shahnaz Perveen、Abdul Hameed、Nor Hadiani Ismail、Muhammad Riaz、Abdul WadoodDOI:10.1016/j.bioorg.2016.08.010日期:2016.10Current study based on the synthesis of new thiazole derivatives via "one pot" multicomponent reaction, evaluation of their in vitro α-glucosidase inhibitory activities, and in silico studies. All synthetic compounds were fully characterized by (1)H NMR, (13)C NMR and EIMS. CHN analysis was also performed. These newly synthesized compounds showed activities in the range of IC50=9.06±0.10-82.50±1.70μM当前的研究基于通过“一锅”多组分反应合成新的噻唑衍生物,评估其体外α-葡萄糖苷酶抑制活性以及计算机模拟研究。所有合成化合物均通过(1)H NMR,(13)C NMR和EIMS进行了全面表征。还进行了CHN分析。与标准阿卡波糖(IC50 = 38.25±0.12μM)相比,这些新合成的化合物显示的活性在IC50 = 9.06±0.10-82.50±1.70μM范围内。值得一提的是大多数化合物,例如1(IC50 = 23.60±0.39μM),2(IC50 = 22.70±0.60μM),3(IC50 = 22.40±0.32μM),4(IC50 = 26.5±0.40μM) ,6(IC50 = 34.60±0.60μM),7(IC50 = 26.20±0.43μM),8(IC50 = 14.06±0.18μM),9(IC50 = 17.60±0.28μM),10(IC50 = 27.16±0
-
Synthesis of novel 5-(aroylhydrazinocarbonyl)escitalopram as cholinesterase inhibitors作者:Mehr-un Nisa、Munawar A. Munawar、Amber Iqbal、Asrar Ahmed、Muhammad Ashraf、Qurra-tul-Ann A. Gardener、Misbahul A. KhanDOI:10.1016/j.ejmech.2017.06.036日期:2017.9A novel series of 5-(aroylhydrazinocarbonyl)escitalopram (58–84) have been designed, synthesized and tested for their inhibitory potential against cholinesterases. 3-Chlorobenzoyl- (71) was found to be the most potent compound of this series having IC50 1.80 ± 0.11 μM for acetylcholinesterase (AChE) inhibition. For the butyrylcholinesterase (BChE) inhibition, 2-bromobenzoyl- (76) was the most active已经设计,合成和测试了一系列新型的5-(芳酰基肼基羰基)依他普仑(58-84)对胆碱酯酶的抑制潜力。发现3-氯苯甲酰基- (71)是该系列中最有效的化合物,对乙酰胆碱酯酶(AChE)的抑制作用的IC 50为1.80±0.11μM。对于丁酰胆碱酯酶(BChE)抑制,2-溴苯甲酰基-(76)是该系列中活性最高的化合物,IC 50为2.11±0.31μM。构效关系说明温和的给电子基团增强了酶的抑制作用,而吸电子基团降低了除o -NO 2以外的抑制。然而,取代基的大小和位置影响酶抑制。。在AChE的对接研究中,配体71、72和76分别显示5874、5756和5666以及ACE的得分分别为-64.92,-203.25和-140.29 kcal / mol。在BChE的情况下,配体71、76和81分别显示出ACE值为-170.91,-256.84和-235.97 kcal / mol的高分6016、6150和5994。
表征谱图
-
氢谱1HNMR
-
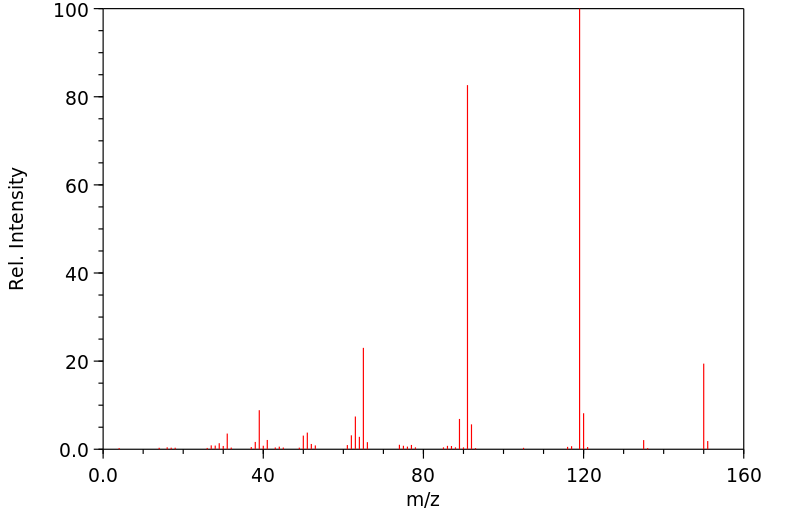
质谱MS
-
碳谱13CNMR
-
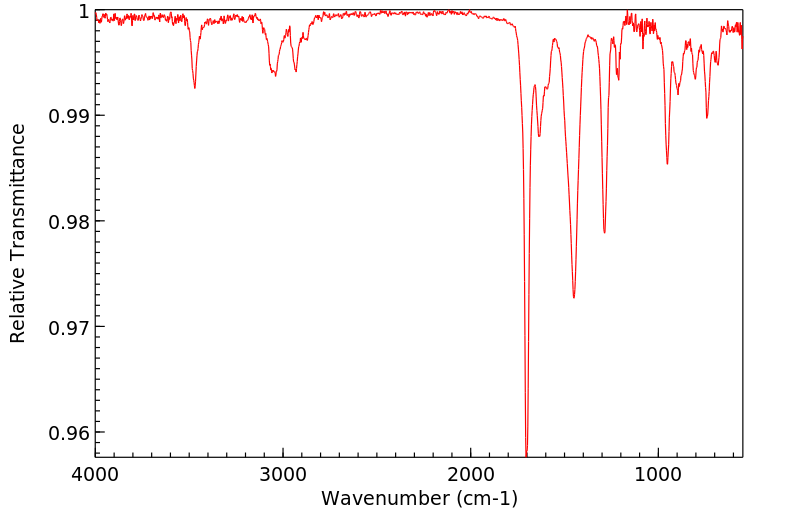
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫