6,8-二氯-3-甲酰色酮 | 64481-10-3
物质功能分类
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:169-174 °C
-
沸点:396.7±42.0 °C(Predicted)
-
密度:1.669±0.06 g/cm3(Predicted)
-
稳定性/保质期:
在常温常压下稳定,应远离氧化剂。
计算性质
-
辛醇/水分配系数(LogP):2.5
-
重原子数:15
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:43.4
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险品标志:Xi
-
安全说明:S26,S37/39
-
危险类别码:R36/37/38
-
海关编码:2914700090
-
WGK Germany:3
-
危险性防范说明:P264,P280,P302+P352+P332+P313+P362+P364,P305+P351+P338+P337+P313
-
危险性描述:H315,H319
-
储存条件:请将存放在密封容器内,并置于阴凉、干燥处。
SDS
| Name: | 6 8-Dichloro-3-formylchromone Material Safety Data Sheet |
| Synonym: | 6,8-Dichloro-4-oxo-4H-1-benzopyran-3-carboxaldehyde |
| CAS: | 64481-10-3 |
Synonym:6,8-Dichloro-4-oxo-4H-1-benzopyran-3-carboxaldehyde
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 64481-10-3 | 6,8-Dichloro-3-formylchromone | 97 | unlisted |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.
Potential Health Effects
Eye:
Causes eye irritation. May cause chemical conjunctivitis.
Skin:
Causes skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. May be harmful if swallowed.
Inhalation:
Causes respiratory tract irritation. May be harmful if inhaled.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Minimize dust generation and accumulation. Avoid breathing dust, vapor, mist, or gas. Avoid contact with eyes, skin, and clothing.
Keep container tightly closed. Avoid ingestion and inhalation. Use with adequate ventilation. Wash clothing before reuse.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 64481-10-3: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: brown - yellow
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 174 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature: > 300 deg C
Solubility in water:
Specific Gravity/Density:
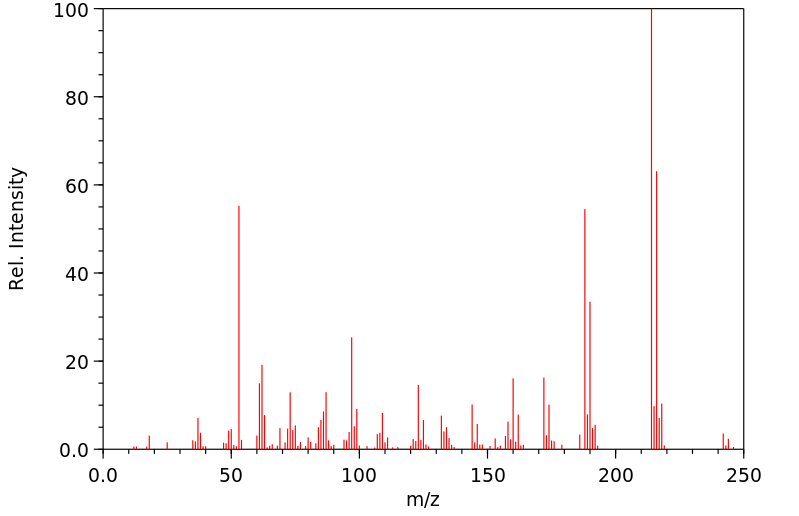
Molecular Formula: C10H4Cl2O3
Molecular Weight: 243.05
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Hydrogen chloride, carbon monoxide, carbon dioxide.
Hazardous Polymerization: Will not occur.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 64481-10-3 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
6,8-Dichloro-3-formylchromone - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
IMO
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
RID/ADR
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing group:
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 37/39 Wear suitable gloves and eye/face
protection.
WGK (Water Danger/Protection)
CAS# 64481-10-3: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 64481-10-3 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 64481-10-3 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 6,8-Dichloro-4-oxo-4H-chromene-3-carboxylic acid 72798-31-3 C10H4Cl2O4 259.045 6,8-二氯-3-氰基色酮 6,8-dichloro-4-oxo-4H-1-benzopyran-3-carbonitrile 72798-32-4 C10H3Cl2NO2 240.045 —— Ethyl 6,8-dichloro-4-oxochromene-3-carboxylate 773136-15-5 C12H8Cl2O4 287.099 —— (E)-3-(4-nitrostyryl)-6, 8-dichloro-4H-chromen-4-one —— C17H9Cl2NO4 362.169 —— 6,8-dichloro-2-oxo-2H-chromene-3-carbonitrile —— C10H3Cl2NO2 240.045 —— 5-(6,8-dichloro-4-oxo-4H-chromen-3-ylmethylene)-2-thioxothiazolidin-4-one 1367578-85-5 C13H5Cl2NO3S2 358.226 —— 5-(6,8-dichloro-4-oxo-4H-chromen-3-ylmethylene)-3-ethyl-2-thioxothiazolidin-4-one 1025227-15-9 C15H9Cl2NO3S2 386.279 —— 3-allyl-5-(6,8-dichloro-4-oxo-4H-chromen-3-ylmethylene)-2-thioxothiazolidin-4-one 433246-17-4 C16H9Cl2NO3S2 398.29
反应信息
-
作为反应物:描述:6,8-二氯-3-甲酰色酮 在 zinc(II) chloride 作用下, 以 二甲基亚砜 为溶剂, 反应 2.0h, 生成 dimethyl 3-(3,5-dichloro-2-hydroxybenzoyl)-9-methoxy-7,12-dihydroindolo[2,3-a]quinolizine-1,12b(6H)-dicarboxylate参考文献:名称:级联合成路线到Centrocountins摘要:在一锅中通过多个步骤进行并包括多个键形成的级联反应和多米诺骨反应是快速有效生成复杂分子结构(包括多种复杂天然产物的支架)的有前途的方法。我们描述了各种单罐级联反应序列的发展,以产生出百里香素,它们是具有许多多环生物碱的基本骨架的四环吲哚衍生物。对以现成的炔烃和3-甲酰基色酮为起始原料的序列进行的机理研究表明,这一一锅法合成过程至少要进行十二次连续转化,并包括至少九种不同的化学反应,这使其成为已知的最长的级联反应序列。日期。DOI:10.1002/chem.201203714
-
作为产物:参考文献:名称:Shindalkar; Madje; Hangarge, Indian Journal of Chemistry - Section B Organic and Medicinal Chemistry, 2005, vol. 44, # 11, p. 2409 - 2411摘要:DOI:
文献信息
-
Synthesis, Molecular Docking and Mosquitocidal Efficacy of Lawsone and its Derivatives Against the Dengue Vector Aedes aegypti L. (Diptera: Culicidae)作者:Antony Stalin、Paul Dhivya、Ding Lin、Yue Feng、Antony Cruz Asharaja、Munusamy Rajiv Gandhi、Balakrishnan Senthamarai Kannan、Subramani Kandhasamy、Appadurai Daniel Reegan、Yuan ChenDOI:10.2174/1573406417666210727121654日期:2022.2
Background: Aedes aegypti is the primary dengue vector, a significant public health problem in many countries. Controlling the growth of Ae. aegypti is the biggest challenge in the mosquito control program, and there is a need for finding bioactive molecules to control Ae. aegypti in order to prevent dengue virus transmission.
Objective: To assess the mosquitocidal property of lawsone and its 3-methyl-4H-chromen-3-yl-1-phenylbenzo[6,7]chromeno[2,3,c]pyrazole-dione derivatives (6a-6h) against various life stages of Ae. aegypti. Besides, to study the mode of action of the active compound by molecular docking and histopathological analysis.
Methods: All derivatives were synthesized from the reaction between 2-hydroxy-1,4-naphthoquinone, chromene-3-carbaldehyde, and 1-phenyl-3-methyl-pyrazol-5-one by using one pot sequential multicomponent reaction. The mosquito life stages were subjected to diverse concentrations ranging from 1.25, 2.5, 5.0, and 10 ppm for lawsone and its derivatives. The structure of all synthesized compounds was characterized by spectroscopic analysis. Docking analysis was performed using autodock tools. Midgut sections of Ae. aegypti larvae were analyzed for histopathological effects.
Results: Among the nine compounds screened, derivative 6e showed the highest mortality on Ae. aegypti life stages. The analyzed LC50 and LC90 results of derivative 6e were 3.01, 5.87 ppm, and 3.41, 6.28 ppm on larvae and pupae of Ae. aegypti, respectively. In the ovicidal assay, the derivative 6e recorded 47.2% egg mortality after 96-hour post-exposure to 10 ppm concentration. In molecular docking analysis, the derivative 6e confirmed strong binding interaction (-9.09 kcal/mol and -10.17 kcal/mol) with VAL 60 and HIS 62 of acetylcholinesterase 1 (AChE1) model and LYS 255, LYS 263 of kynurenine aminotransferase of Ae. aegypti, respectively. The histopathological results showed that the derivative 6e affected the columnar epithelial cells (CC) and peritrophic membrane (pM).
Conclusion: The derivative 6e is highly effective in the life stages of Ae. aegypti mosquito and it could be used in the integrated mosquito management programme.
背景: 埃及伊蚊是登革热的主要传播媒介,在许多国家都是一个重大的公共卫生问题。控制埃及伊蚊的增长是蚊虫控制计划中最大的挑战,需要寻找生物活性分子来控制埃及伊蚊,以防止登革热病毒的传播。 目标: 评估劳森和其3-甲基-4H-香豆素-3-基-1-苯基苯并[6,7]香豆并[2,3,c]吡唑烷二酮衍生物(6a-6h)对埃及伊蚊各个生活阶段的杀蚊活性。此外,通过分子对接和病理学分析研究活性化合物的作用方式。 方法: 所有衍生物都是由2-羟基-1,4-萘醌、香豆素-3-甲醛和1-苯基-3-甲基-吡唑-5-酮通过一锅法连续多组分反应合成的。将蚊子生活阶段暴露于不同浓度的劳森及其衍生物,浓度范围为1.25、2.5、5.0和10 ppm。所有合成化合物的结构都通过光谱分析进行了表征。使用AutoDock工具进行了对接分析。对埃及伊蚊幼虫的中肠部分进行了病理学效果分析。 结果: 在筛选的九种化合物中,衍生物6e对埃及伊蚊生活阶段的杀伤力最高。衍生物6e对埃及伊蚊幼虫和蛹的LC50和LC90结果分别为3.01、5.87 ppm和3.41、6.28 ppm。在杀卵实验中,衍生物6e在10 ppm浓度下暴露96小时后记录到47.2%的卵死亡率。在分子对接分析中,衍生物6e与乙酰胆碱酯酶1(AChE1)模型的VAL 60和HIS 62以及埃及伊蚊的色氨酸胺转移酶的LYS 255、LYS 263显示出强烈的结合作用(-9.09 kcal/mol和-10.17 kcal/mol)。病理学结果显示,衍生物6e影响了柱状上皮细胞(CC)和围食膜(pM)。 结论: 衍生物6e对埃及伊蚊蚊子的生活阶段非常有效,它可以用在综合蚊虫管理计划中。 -
SUBSTITUTED INDOLO [2,3-A] QUINOLIZINES申请人:Waldmann Herbert公开号:US20120316192A1公开(公告)日:2012-12-13The present invention relates to novel substituted indolo[2,3-a]quinolizines and stereoisomeric forms thereof and/or pharmaceutically acceptable salts of these compounds as well as pharmaceutical compositions containing at least one of these substituted indolo[2,3-a]quinolizines together with pharmaceutically acceptable carrier, excipient and/or diluents. Said novel substituted indolo[2,3-a]quinolizines have been identified as useful for the prophylaxis and treatment of cancer by the induction of strong mitotic delays, chromosomal misalignments and mitotic tri- and multipolarization leading to cell cycle stop and apoptosis. Furthermore a synthesis for preparation of the substituted indolo[2,3-a]quinolizines is disclosed in the present invention.
-
SUBSTITUTED 6, 7-DIALKOXY-3-ISOQUINOLINE DERIVATIVES AS INHIBITORS OF PHOSPHODIESTERASE 10 (PDE 10A)申请人:Allergan, Inc.公开号:US20150158895A1公开(公告)日:2015-06-11The invention relates to compounds of the formula or a pharmaceutically acceptable salt thereof, wherein R′, R 1 through R 7 and Ar are as defined herein. These compounds are useful as inhibitors of phosphodiesterase 10 (PDE10A) which are useful in treating central nervous system diseases such as psychosis and also in treating, for example, obesity, type II diabetes, metabolic syndrome, glucose intolerance, pain and ophthalmic diseases.
-
Photoredox Functionalization of 3-Halogenchromones, 3-Formylchromones, and Chromone-3-carboxylic Acids: Routes to 3-Acylchromones作者:Satenik Mkrtchyan、Viktor O. IaroshenkoDOI:10.1021/acs.joc.0c00537日期:2020.6.5describes a set of new and efficient synthetic routes toward 3-acyl-substituted chromones ranging from readily available chromone precursors, namely 3-halogenchromones, 3-formylchromones, and chromone-3-carboxylic acids by means of visible-light photoredox catalysis. The operationally simple protocols transform a wide variety of chromone derivatives into challenging 3-acyl-substituted chromones in excellent
-
Multi-component cascade reaction of 3-formylchromones: highly selective synthesis of 4,5-dihydro-[4,5′-bipyrimidin]-6(1<i>H</i>)-one derivatives作者:Li Chen、Rong Huang、Xing-Han Yun、Tian-Hui Hao、Sheng-Jiao YanDOI:10.1039/d1cc02437j日期:——5′-bipyrimidin]-6(1H)-ones (DBPMOs) 4 was constructed regioselectively in suitable to excellent yields. Moreover, intermediates 4 then underwent a novel, metal- and oxidant-free cascade reaction to produce a series of [4,5′-bipyrimidin]-6(1H)-ones (BPMOs) 5. The formation of the bipyrimidine derivatives 4–5 was enabled by the formation of five bonds and the cleavage of one bond in one pot. This protocol can be开发了一种通过有趣且相当复杂的多组分级联反应从 3-甲酰基色酮、2-(吡啶-2-基)乙酸乙酯衍生物和脒盐酸盐构建高度官能化的双嘧啶衍生物 4 和 5 的新方案. 通过将三种底物在乙腈或 DMF 中的混合物与 Cs 2 CO 3一起回流来证明级联反应。一系列 4,5-二氢-[4,5'-联嘧啶]-6(1 H )-ones (DBPMOs) 4 被区域选择性地构建,以达到优异的产率。此外,中间体 4 随后经历了一种新型的、无金属和无氧化剂的级联反应,产生了一系列 [4,5'-联嘧啶]-6(1 H)-ones (BPMOs) 5. 联嘧啶衍生物4-5的形成是通过形成五个键并在一锅中裂解一个键来实现的。该协议可用于通过多组分一锅级联反应而不是多步反应合成功能化双嘧啶衍生物,适用于双嘧啶衍生物的组合和平行合成。
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
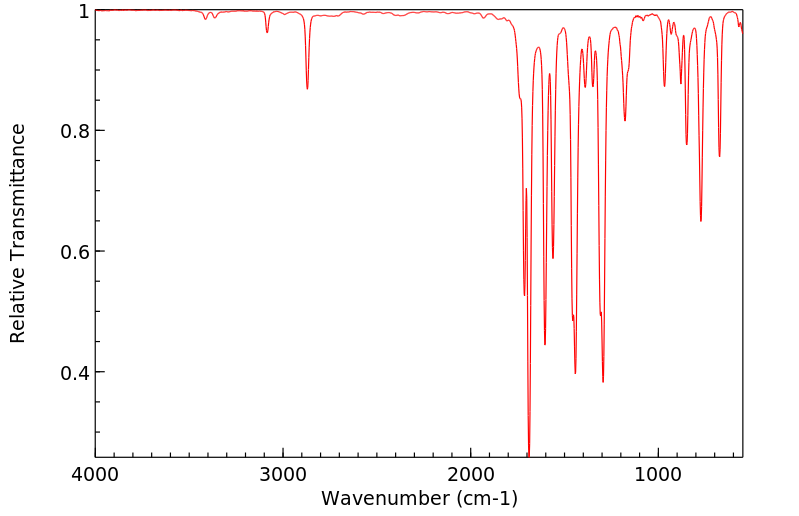
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息