双(2-噻吩基)二硫醚 | 6911-51-9
中文名称
双(2-噻吩基)二硫醚
中文别名
2,2'-二噻吩基二硫醚;2-噻吩基二硫;2-噻吩基二硫化物;二噻吩二硫;2-噻吩二硫醚;二噻吩二硫醚;二噻吩基二硫醚
英文名称
2-thienyl disulfide
英文别名
1,2-di(thiophen-2-yl)disulfane;bis(2-thienyl)disulfide;dithiophen-2-yl disulfide;di(2-thienyl)disulfide;bis(thiophen-2-yl)disulfide;1,2-di(thiophen-2-yl)disulfide;2,2'-dithienyl disulfide;(dithien-2-yl)disulfide;2-(thiophen-2-yldisulfanyl)thiophene
CAS
6911-51-9
化学式
C8H6S4
mdl
MFCD00066333
分子量
230.4
InChiKey
YOLFWWMPGNMXFI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:55-60 °C(lit.)
-
沸点:132°C/0.1mmHg(lit.)
-
密度:1.45±0.1 g/cm3(Predicted)
-
LogP:3.77
-
物理描述:Solid
-
溶解度:5% in CH3CN
-
保留指数:1871
计算性质
-
辛醇/水分配系数(LogP):4.1
-
重原子数:12
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:107
-
氢给体数:0
-
氢受体数:4
安全信息
-
危险品标志:Xn
-
安全说明:S22,S26,S36/37,S39
-
危险类别码:R20/21/22
-
WGK Germany:3
-
海关编码:2934999090
-
危险品运输编号:2811
-
RTECS号:JO1987000
-
包装等级:III
-
危险类别:9
-
危险性防范说明:P280,P305+P351+P338,P273
-
危险性描述:H302,H318,H410
-
储存条件:存放于惰性气体中,以避免与空气接触。
SDS
模块 1. 化学品
1.1 产品标识符
: 2-噻吩基二硫
产品名称
1.2 鉴别的其他方法
Dithienyl disulfide
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅用于研发。不作为药品、家庭或其它用途。
模块 2. 危险性概述
2.1 GHS-分类
急性毒性, 经口 (类别 4)
严重眼睛损伤 (类别 1)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 危险
危险申明
H302 吞咽有害。
H318 造成严重眼损伤。
警告申明
预防措施
P264 操作后彻底清洁皮肤。
P270 使用本产品时不要进食、饮水或吸烟。
P280 穿戴防护手套/ 眼保护罩/ 面部保护罩。
事故响应
P301 + P312 如果吞咽并觉不适: 立即呼叫解毒中心或就医。
P305 + P351 + P338 如与眼睛接触,用水缓慢温和地冲洗几分钟。如戴隐形眼镜并可方便地取
出,取出隐形眼镜,然后继续冲洗.
P310 立即呼叫中毒控制中心或医生.
P330 漱口。
废弃处置
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物
恶臭
模块 3. 成分/组成信息
3.1 物 质
: Dithienyl disulfide
别名
: C8H6S4
分子式
: 230.39 g/mol
分子量
组分 浓度或浓度范围
2-Thienyl disulfide
<=100%
化学文摘登记号(CAS 6911-51-9
No.)
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 向到现场的医生出示此安全技术说明书。
吸入
如果吸入,请将患者移到新鲜空气处。 如呼吸停止,进行人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用大量水彻底冲洗至少15分钟并请教医生。
食入
切勿给失去知觉者通过口喂任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
恶心, 头痛, 呕吐, 据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,抗乙醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 硫氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 作业人员防护措施、防护装备和应急处置程序
使用个人防护用品。 避免粉尘生成。 避免吸入蒸气、烟雾或气体。 保证充分的通风。
人员疏散到安全区域。 避免吸入粉尘。
6.2 环境保护措施
不要让产品进入下水道。
6.3 泄漏化学品的收容、清除方法及所使用的处置材料
收集和处置时不要产生粉尘。 扫掉和铲掉。 放入合适的封闭的容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 避免形成粉尘和气溶胶。
在有粉尘生成的地方,提供合适的排风设备。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 使容器保持密闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
根据良好的工业卫生和安全规范进行操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
面罩與安全眼鏡请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
全套防化学试剂工作服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和数量来选择。
呼吸系统防护
如危险性评测显示需要使用空气净化的防毒面具,请使用全面罩式多功能微粒防毒面具N100型(US
)或P3型(EN
143)防毒面具筒作为工程控制的候补。如果防毒面具是保护的唯一方式,则使用全面罩式送风防毒
面具。 呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 固体
b) 气味
恶臭
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: 55 - 60 °C - lit.
f) 沸点、初沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 蒸汽密度
无数据资料
m) 密度/相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应
无数据资料
10.4 应避免的条件
无数据资料
10.5 不相容的物质
强氧化剂, 强碱
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
半数致死剂量 (LD50) 经口 - 小鼠 - 400 mg/kg
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞致突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 误吞对人体有害。
皮肤 通过皮肤吸收可能有害。 可能引起皮肤刺激。
眼睛 引起眼睛灼伤。
接触后的征兆和症状
恶心, 头痛, 呕吐, 据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: JO1987000
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久性和降解性
无数据资料
12.3 潜在的生物累积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不良影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和不可回收的溶液交给有许可证的公司处理。
与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
受污染的容器和包装
按未用产品处置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: 3335
14.2 联合国运输名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: Aviation regulated solid, n.o.s. (2-Thienyl disulfide)
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: 9
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: III
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 国际空运危规: 否
海洋污染物(是/否): 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
双(2-噻吩基)二硫醚是一种二硫醚,作为重要有机试剂和合成中间体,在有机合成、高分子等领域有着广泛应用。
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-phenyldithio-thiophene 93336-13-1 C10H8S3 224.372
反应信息
-
作为反应物:描述:双(2-噻吩基)二硫醚 在 copper(l) iodide 、 1,10-菲罗啉 、 potassium carbonate 作用下, 以 水 、 二甲基亚砜 为溶剂, 以76%的产率得到2-(2-噻吩基硫)噻吩参考文献:名称:芳基二硫属元素化物的脱卤代反应通过铜催化合成芳基硫属元素化物摘要:公开了一种通过铜催化将芳族二硫属元素进行脱硫反应以合成芳基硫属元素化物的应用。实际条件,广泛的底物范围以及对几个敏感基团(如醛,酮,酯,酰胺,氰化物,烯烃,硝基和甲基磺酰基)的良好官能团耐受性突出了该方法。此外,该方法的鲁棒性通过雌酮的后期修饰和伏替西汀的合成来描述。值得注意的是,通过该方案成功地完成了在较温和条件下具有较大环张力的更具挑战性的有机材料的合成,以及某些不能通过金属催化的芳基卤素偶合反应合成的含卤素的二芳基硫醚的合成。DOI:10.1021/acscatal.9b04931
-
作为产物:描述:2-溴噻吩 在 potassium sulfide 、 nickel(II) chloride hexahydrate 、 乙酰丙酮 、 potassium hydroxide 作用下, 以 水 、 N,N-二甲基甲酰胺 为溶剂, 反应 24.0h, 以51%的产率得到双(2-噻吩基)二硫醚参考文献:名称:以硫化钾为硫源的镍催化对称合成二硫化物摘要:摘要已经描述了一种从芳基和烷基卤化物和硫化钾合成有机二硫化物的有效的一锅法。K 2 S充当廉价且易于获得的硫源。使用NiCl 2 ·6H 2 O和乙酰丙酮作为催化体系,以良好至极好的收率制备了各种对称的二芳基二芳基和二烷基二硫化物。 图形概要DOI:10.1007/s00706-016-1673-2
-
作为试剂:描述:参考文献:名称:Free-Radical Addition of Heteroarenethiols and Heteroarylmethanethiols to Hexyne and Phenylacetylene. Chemical Behavior of the Transient .beta.-Sulfanylvinyl Radicals摘要:The free-radical reaction of a number of heteroarenethiols (including 2-thiophene-, 2-benzo[b]furan-and 2-benzo[b]thiophenethiol) and heteroarylmethanethiols (including 2-furyl-, 2-thienyl-, and 3-thienylmethanethiol) with hex-1-yne and phenylacetylene has been investigated in benzene solution both at 100 degrees C in the presence of AIBN and at room temperature in the presence of BEt(3)/O-2. Under both of these conditions the above thiols generally furnished transient 2-sulfanylvinyl radicals through regioselective addition of corresponding sulfanyl radicals to the terminal alkyne carbon, but 2-benzo[b]furanthiol failed to react with either alkyne in the presence of BEt(3)/O-2 and unexpectedely gave 2-(ethylsulfanyl)benzo[b]furan to a significant extent. The produced 2-(2-heteroarylsulfanyl) vinyl radicals largely preferred to undergo intermolecular H-abstraction reaction rather than intramolecular 5-endo cyclization onto the heteroaryl moiety of the 2-sulfanyl substituent. The 2-[(2-thienylmethyl)sulfanyl]- and, especially, 2-[(2-furylmethyl)sulfanyl]vinyl radicals, besides H-abstraction, promptly underwent intramolecular B-exo cyclization to give spiro radicals that interestingly underwent beta-scission of their respective C-S and C-O bond resulting in ring cleavage of the original heteroaryl group. The 2-[(3-thienylmethyl)sulfanyl]vinyl radicals did not exhibit any similar B-exo cyclization, but did undergo a 6-endo cyclization to a very slight extent.DOI:10.1021/jo00129a039
文献信息
-
水相中催化分子氧氧化生成具有S-S键的二硫 化合物的方法
-
An Organodiselenide with Dual Mimic Function of Sulfhydryl Oxidases and Glutathione Peroxidases: Aerial Oxidation of Organothiols to Organodisulfides作者:Vandana Rathore、Aditya Upadhyay、Sangit KumarDOI:10.1021/acs.orglett.8b02756日期:2018.10.5peroxidase (GPx) enzymes for oxidation of thiols by oxygen and hydrogen peroxide, respectively, into disulfides has been presented. The developed catalyst oxidizes an array of organothiols into respective disulfides in practical yields by using aerial O2 to avoid any reagents/additives, base, and light source. The synthesized diselenide also catalyzes the reduction of hydrogen peroxide into water by following
-
Copper-catalyzed <i>ortho</i>-selective direct sulfenylation of <i>N</i>-aryl-7-azaindoles with disulfides
-
Sequential C–H activation enabled expedient delivery of polyfunctional arenes作者:Wensen Ouyang、Xiaoqing Cai、Xiaojian Chen、Jie Wang、Jianhang Rao、Yang Gao、Yanping Huo、Qian Chen、Xianwei LiDOI:10.1039/d1cc03243g日期:——Modular construction of polyfunctional arenes from abundant feedstocks stands as an unremitting pursue in synthetic chemistry, accelerating the discovery of drugs and materials. Herein, using the multiple C–H activation strategy with versatile imidate esters, the expedient delivery of molecular libraries of densely functionalized sulfur-containing arenes was achieved, which enabled the concise construction
-
Transition-Metal-Free C–N Bond Activation: Synthesis of α,α-Bis(arylthio) Aldehydes作者:Chen-Liang Deng、Xu-Hong Gao、Wei Xu、Xing-Guo ZhangDOI:10.1055/s-0036-1588135日期:——bond activation of tertiary amines and subsequent aryl thiolation has been explored. A variety of disulfides and series of tertiary amines were efficiently converted into the corresponding α,α-bis(arylthio) aldehydes in moderate to good yields. The reaction proceeded well under transition-metal-free conditions via a C–N activation process.
表征谱图
-
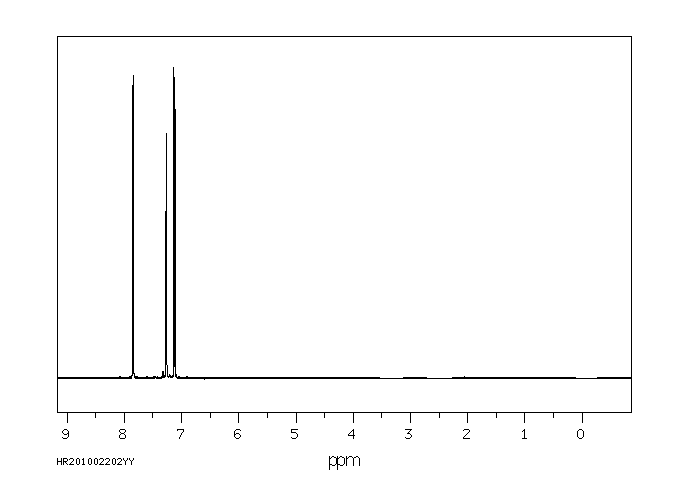
氢谱1HNMR
-
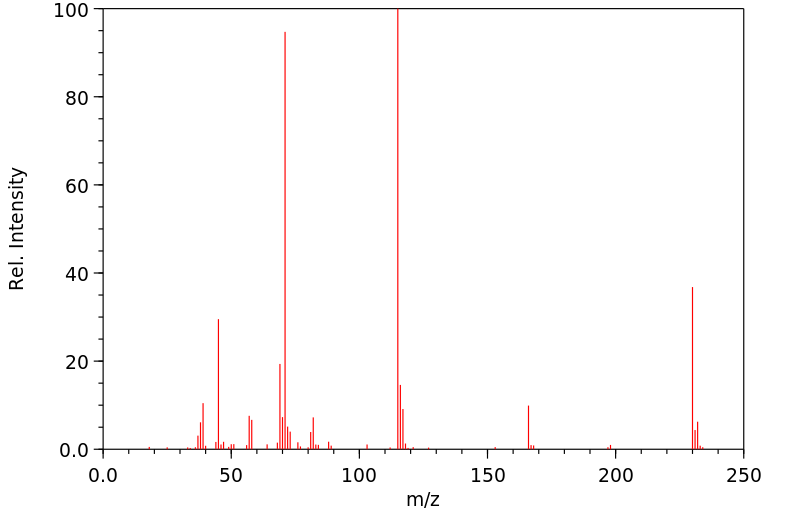
质谱MS
-
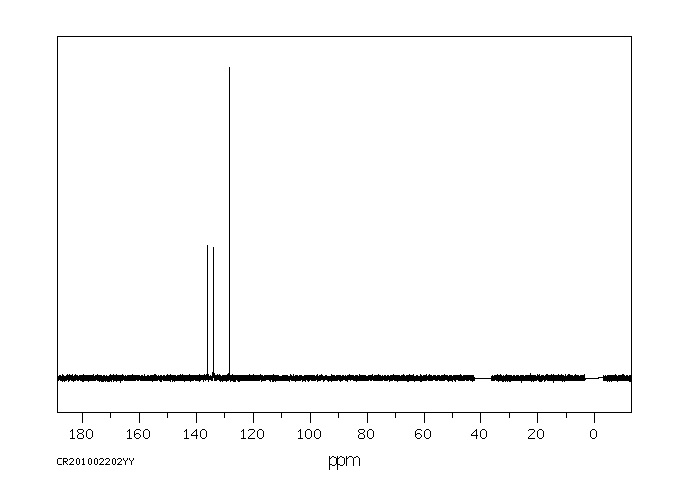
碳谱13CNMR
-
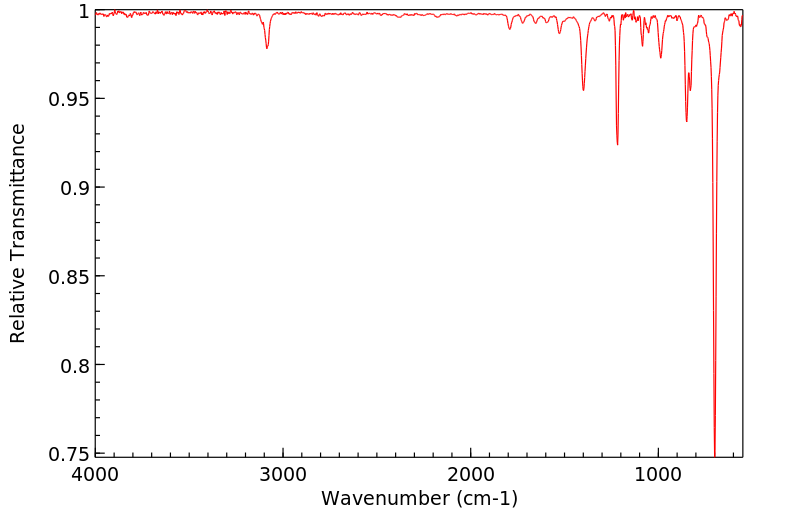
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
香薷二醇
顺式-1-(2-呋喃基)-1-戊烯
顺-1,2-二氰基-1,2-双(2,4,5-三甲基-3-噻吩基)乙烯
顺-1,2-(2-噻嗯基)二乙烯
雷尼替丁-N,S-二氧化物
雷尼替丁-N-氧化物
钴(II)双[(2-吡啶基甲基)(叔丁基二甲基甲硅烷基)酰胺]
西拉诺德
螺[环氧乙烷-2,3'-吡咯并[1,2-a]吡嗪]
萘并[2,1,8-def]喹啉
苯硫基溴化镁
苯甲酸,2-[[[7-[[(3.β.)-3-羟基-28-羰基羽扇-20(29)-烯-28-基]amino]庚基]氨基]羰基]
苍术素
羟胺,O-[4-(2-呋喃基)丁基]-
缩水甘油糠醚
紫苏烯
糠醛肟
糠醛氰醇的1-乙氧基乙基醚
糠醇-d2
糠醇
糠基硫醇-d2
糠基硫醇
糠基甲基硫醚
糠基氯
糠基氨基甲酸异丙酯
糠基丙基醚
糠基丙基二硫醚
糠基3-巯基-2-甲基丙酸酯
糠基-异戊基醚
糠基-异丁基醚
糠基 2-甲基-3-呋喃基二硫醚
磷杂茂
碘化N,N,N-三甲基丁烷-1-铵
硫酸异丙基糠酯
硫代磷酸O-糠基O-甲基S-(2-丙炔基)酯
硫代磷酸O-乙基O-糠基S-(2-丙炔基)酯
硫代甲酸S-糠酯
硫代噻吩甲酰基三氟丙酮
硫代乙酸糠酯
硫代丙酸糠酯
硒吩-3-羧酸酰肼
硅烷,三(1-甲基乙基)[(3-甲基-2-呋喃基)氧代]-
硅烷,[2-(3-呋喃基)乙烯基]三甲基-,(E)-
硅烷,(1,1-二甲基乙基)(2-呋喃基甲氧基)二甲基-
砷杂苯
甲酸糠酯
甲氧亚胺基呋喃乙酸铵盐
甲基糠基醚
甲基糠基二硫
甲基呋喃-2-基甲基氨基甲酸酯