N-甲基异丁基胺 | 625-43-4
中文名称
N-甲基异丁基胺
中文别名
N-甲基异丁胺;N-甲基异丙基胺
英文名称
2-methylpropyl(methyl)amine
英文别名
isobutylmethylamine;N-methylisobutylamine;N‐methylisobutylamine;N,2-dimethylpropan-1-amine
CAS
625-43-4
化学式
C5H13N
mdl
MFCD00015043
分子量
87.1649
InChiKey
QKYWADPCTHTJHQ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-75°C (estimate)
-
沸点:78 °C
-
密度:0,73 g/cm3
-
保留指数:635
-
稳定性/保质期:
- 远离氧化物和酸。
- 它存在于香料烟烟叶及烟气中。
计算性质
-
辛醇/水分配系数(LogP):1.1
-
重原子数:6
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:12
-
氢给体数:1
-
氢受体数:1
安全信息
-
TSCA:Yes
-
危险等级:3
-
安全说明:S26,S36/37/39
-
危险品运输编号:UN 2733
-
海关编码:2921199090
-
危险类别码:R34,R11
-
包装等级:II
-
危险类别:3,8
-
危险性防范说明:P210,P233,P235,P240,P241,P242,P243,P260,P264,P280,P301+P330+P331,P303+P361+P353,P304+P340,P305+P351+P338,P310,P321,P363,P370+P378,P403+P235,P405,P501
-
危险性描述:H225,H314
-
储存条件:应将化学物质存放在密封容器中,并放置在阴凉、干燥的地方。储存地点需上锁,钥匙由技术人员及其助手保管。同时,存储区域要远离氧化剂,并避免与酸类物质混放。
SDS
N-甲基异丁胺 修改号码:5
模块 1. 化学品
产品名称: N-Methylisobutylamine
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第2级
健康危害
皮肤腐蚀/刺激 1B类
严重损伤/刺激眼睛 第1级
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 危险
危险描述 高度易燃液体和蒸气
造成严重的皮肤灼伤和眼损伤
防范说明
[预防] 远离热源/火花/明火/热表面。禁烟。
保持容器密闭。
使用防爆的电气/通风/照明设备。采取预防措施以防静电和火花引起的着火。
切勿吸入。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 吸入:将受害者移到新鲜空气处,在呼吸舒适的地方保持休息。
食入:漱口。切勿催吐。
眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
皮肤接触:立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
被污染的衣物清洗后方可重新使用。
立即呼叫解毒中心/医生。
[储存] 存放于通风良好处。保持凉爽。
存放处须加锁。
N-甲基异丁胺 修改号码:5
模块 2. 危险性概述
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): N-甲基异丁胺
百分比: >98.0%(GC)
CAS编码: 625-43-4
俗名: N-Isobutylmethylamine
分子式:
C5H13N
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。立即呼叫解毒中心/医生。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
立即呼叫解毒中心/医生。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
立即呼叫解毒中心/医生。
食入: 立即呼叫解毒中心/医生。漱口。切勿引吐。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,大量水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:喷水,保持容器冷却。如果安全,消除一切火源。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用特殊的个人防护用品(自携式呼吸器)。远离溢出物/泄露处并处在上风处。确保
紧急措施: 足够通风。
泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 回收到密闭容器前用干砂或惰性吸收剂吸收泄漏物。一旦大量泄漏,筑堤控制。附着
物或收集物应该根据相关法律法规废弃处置。
副危险性的防护措施 移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防爆设备。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离热源/火花/明火
/热表面。禁烟。采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 如果可能,使用封闭系统。如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
存放于惰性气体环境中。
存放处须加锁。
远离不相容的材料比如氧化剂存放。
气敏
包装材料: 依据法律。
N-甲基异丁胺 修改号码:5
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
个人防护用品
呼吸系统防护: 半面罩或全面罩呼吸器,自携式呼吸器(SCBA),供气呼吸器等。依据当地和政府法
规,使用通过政府标准的呼吸器。
手部防护: 防渗手套。
眼睛防护: 护目镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防渗防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
外形(20°C): 液体
外观: 透明
颜色: 无色-几乎无色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 78 °C
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.73
溶解度:
[水] 混和
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
避免接触的条件: 火花, 明火, 静电
须避免接触的物质 氧化剂, 酸
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx)
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
N-甲基异丁胺 修改号码:5
模块 12. 生态学信息
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧,焚烧时需要特别注
意该物质是高度可燃的。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 第3类 易燃液体 。
副危险性: 第8类 腐蚀品
UN编号: 2733
正式运输名称: 胺类, 易燃的, 腐蚀的, 不另作详细说明
包装等级: II
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: N-Methylisobutylamine
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第2级
健康危害
皮肤腐蚀/刺激 1B类
严重损伤/刺激眼睛 第1级
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 危险
危险描述 高度易燃液体和蒸气
造成严重的皮肤灼伤和眼损伤
防范说明
[预防] 远离热源/火花/明火/热表面。禁烟。
保持容器密闭。
使用防爆的电气/通风/照明设备。采取预防措施以防静电和火花引起的着火。
切勿吸入。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 吸入:将受害者移到新鲜空气处,在呼吸舒适的地方保持休息。
食入:漱口。切勿催吐。
眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
皮肤接触:立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
被污染的衣物清洗后方可重新使用。
立即呼叫解毒中心/医生。
[储存] 存放于通风良好处。保持凉爽。
存放处须加锁。
N-甲基异丁胺 修改号码:5
模块 2. 危险性概述
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): N-甲基异丁胺
百分比: >98.0%(GC)
CAS编码: 625-43-4
俗名: N-Isobutylmethylamine
分子式:
C5H13N
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。立即呼叫解毒中心/医生。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
立即呼叫解毒中心/医生。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
立即呼叫解毒中心/医生。
食入: 立即呼叫解毒中心/医生。漱口。切勿引吐。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,大量水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:喷水,保持容器冷却。如果安全,消除一切火源。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用特殊的个人防护用品(自携式呼吸器)。远离溢出物/泄露处并处在上风处。确保
紧急措施: 足够通风。
泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 回收到密闭容器前用干砂或惰性吸收剂吸收泄漏物。一旦大量泄漏,筑堤控制。附着
物或收集物应该根据相关法律法规废弃处置。
副危险性的防护措施 移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防爆设备。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离热源/火花/明火
/热表面。禁烟。采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 如果可能,使用封闭系统。如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
存放于惰性气体环境中。
存放处须加锁。
远离不相容的材料比如氧化剂存放。
气敏
包装材料: 依据法律。
N-甲基异丁胺 修改号码:5
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
个人防护用品
呼吸系统防护: 半面罩或全面罩呼吸器,自携式呼吸器(SCBA),供气呼吸器等。依据当地和政府法
规,使用通过政府标准的呼吸器。
手部防护: 防渗手套。
眼睛防护: 护目镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防渗防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
外形(20°C): 液体
外观: 透明
颜色: 无色-几乎无色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 78 °C
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.73
溶解度:
[水] 混和
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
避免接触的条件: 火花, 明火, 静电
须避免接触的物质 氧化剂, 酸
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx)
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
N-甲基异丁胺 修改号码:5
模块 12. 生态学信息
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧,焚烧时需要特别注
意该物质是高度可燃的。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 第3类 易燃液体 。
副危险性: 第8类 腐蚀品
UN编号: 2733
正式运输名称: 胺类, 易燃的, 腐蚀的, 不另作详细说明
包装等级: II
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
制备方法与用途
合成制备方法
- 烟草:OR,18。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 N-(2-甲基丙基)甲酰胺 N-isobutylformamide 6281-96-5 C5H11NO 101.148 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1-异丁基-1-甲基肼 1-Isobutyl-1-methylhydrazin 20240-63-5 C5H14N2 102.18
反应信息
-
作为反应物:描述:参考文献:名称:无金属高价碘/TEMPO 介导的胺氧化和对反应途径的机理洞察†摘要:描述了使用 PhI(OAc) 2结合催化量的 TEMPO 作为氧化剂将伯胺和仲胺氧化成相应的醛和酮的高效无金属方法。该协议速度快,可在较温和的反应条件下以优异的收率提供多种产品。此外,光谱学方法很好地证明了机理研究。DOI:10.1039/c8ra07451h
-
作为产物:参考文献:名称:Bowen, Richard D.; Harrison, Alex G.; Reiner, Eric J., Journal of the Chemical Society. Perkin transactions II, 1988, p. 1009 - 1014摘要:DOI:
-
作为试剂:参考文献:名称:Process for the alkylation of secondary aliphatic amines in the presence摘要:提供了一种用于辅助烷基胺烷基化的过程,包括在过渡金属酰胺的存在下,将辅助烷基胺与氨基的α碳原子上至少一个氢反应,与烯烃反应。公开号:US04110377A1
文献信息
-
Catalytic Hydrogenation of Amides to Amines under Mild Conditions作者:Mario Stein、Bernhard BreitDOI:10.1002/anie.201207803日期:2013.2.18Under (not so much) pressure: A general method for the hydrogenation of tertiary and secondary amides to amines with excellent selectivity using a bimetallic Pd–Re catalyst has been developed. The reaction proceeds under low pressure and comparatively low temperature. This method provides organic chemists with a simple and reliable tool for the synthesis of amines.
-
Bequeme Darstellung von reinen N-Methylalkylaminen durch Zink/Salzsäure-Reduktion von 1,3,5-Tris(alkyl)-hexahydro-1,3,5-triazinen作者:Mohammed Al Shaik、Herbert OelschlägerDOI:10.1002/ardp.19843170306日期:——N‐Methyl‐alkylamine können bequem und rasch über die 1,3,5‐Tris(alkyl)‐hexahydro)‐1,3,5‐triazine durch Reduktion mit Zink/Salzsäure bei −5° im Zutropfverfahren gewonnen werden. Die Reinheit (GC) beträgt ∼ 95%.
-
Investigation of the Mechanism of C(sp<sup>3</sup>)−H Bond Cleavage in Pd(0)-Catalyzed Intramolecular Alkane Arylation Adjacent to Amides and Sulfonamides作者:Sophie Rousseaux、Serge I. Gorelsky、Benjamin K. W. Chung、Keith FagnouDOI:10.1021/ja103081n日期:2010.8.11reactivity of C(sp(3))-H bonds adjacent to a nitrogen atom can be tuned to allow intramolecular alkane arylation under Pd(0) catalysis. Diminishing the Lewis basicity of the nitrogen lone pair is crucial for this catalytic activity. A range of N-methylamides and sulfonamides react exclusively at primary C(sp(3))-H bonds to afford the products of alkane arylation in good yields. The isolation of a Pd(II) reaction可以调整与氮原子相邻的 C(sp(3))-H 键的反应性,以允许在 Pd(0) 催化下进行分子内烷烃芳基化。降低氮孤对的路易斯碱度对于这种催化活性至关重要。一系列 N-甲基酰胺和磺酰胺仅在初级 C(sp(3))-H 键上反应,以高产率提供烷烃芳基化产物。Pd(II) 反应中间体的分离使得能够评估反应机理,重点是碱在 C(sp(3))-H 键断裂步骤中的作用。这些化学计量研究的结果,连同动力学同位素效应实验,为协调的金属化-去质子化 (CMD) 过渡态提供了罕见的实验支持,此前已在烷烃 C(sp(3))-H 芳基化中提出了这种过渡态。而且,DFT 计算揭示了新戊酸盐添加剂作为磷化氢从 Pd(II) 中间体解离的促进剂的额外作用,使 CMD 过渡态成为可能。最后,进行了动力学研究,揭示了反应速率表达及其与新戊酸盐浓度的关系。
-
Photocatalytic Intramolecular C–H Amination Using <i>N</i>-Oxyureas as Nitrene Precursors作者:Ryan A. Ivanovich、Dilan E. Polat、André M. BeaucheminDOI:10.1021/acs.orglett.0c02200日期:2020.8.21Nitrenes are remarkable high-energy chemical species that enable direct C–N bond formation, typically via controlled reactions of metal-stabilized nitrenes. Here, in contrast, the combined use of photocatalysis with careful engineering of the precursor enabled C–H amination forming imidazolidinones and related nitrogen heterocycles from readily accessible hydroxylamine precursors. Preliminary mechanistic
-
Novel steroidal pure antiestrogens作者:Jean Bowler、Timothy J. Lilley、John D. Pittam、Alan E. WakelingDOI:10.1016/0039-128x(89)90076-7日期:1989.7A series of steroidal estrogen antagonists with no intrinsic estrogenicity in rat uterotrophic-antiuterotrophic tests has been discovered. The compounds are derivatives of estradiol containing amidoalkyl side chains at the 7 alpha-position. The most potent compounds are N-n-butyl-N-methyl-11-(3,17 beta-dihydroxyestra- 1,3,5(10)-trien-7 alpha-yl) undecanamide and N-2,2,3,3,4,4,4-heptafluorobutyl-N-methyl-11-(3
表征谱图
-
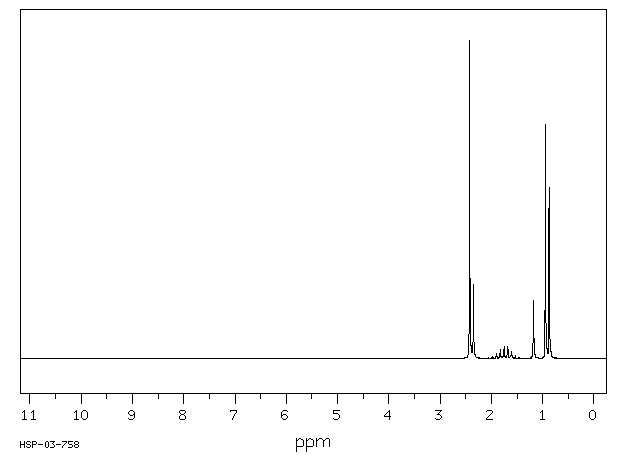
氢谱1HNMR
-
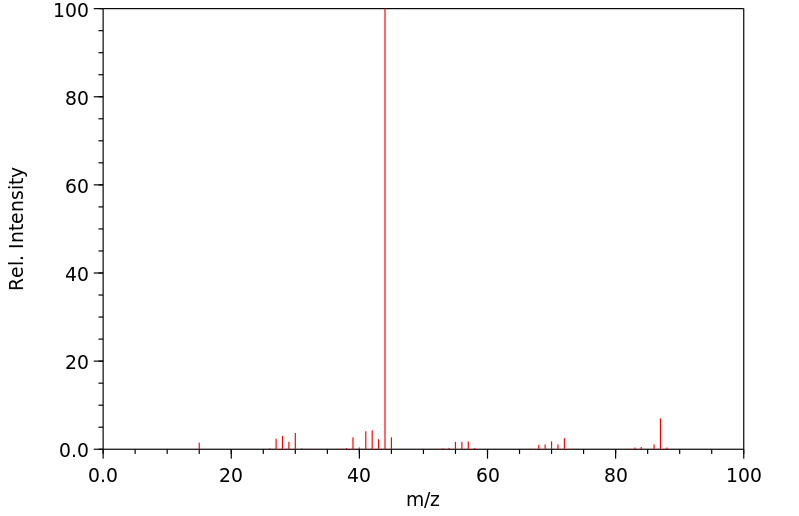
质谱MS
-
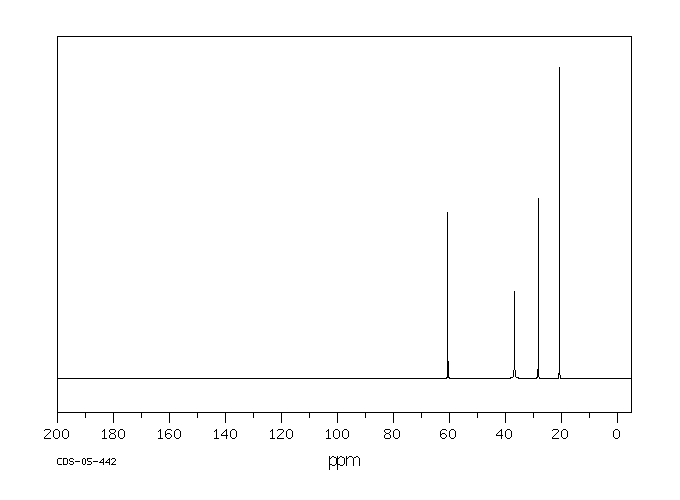
碳谱13CNMR
-
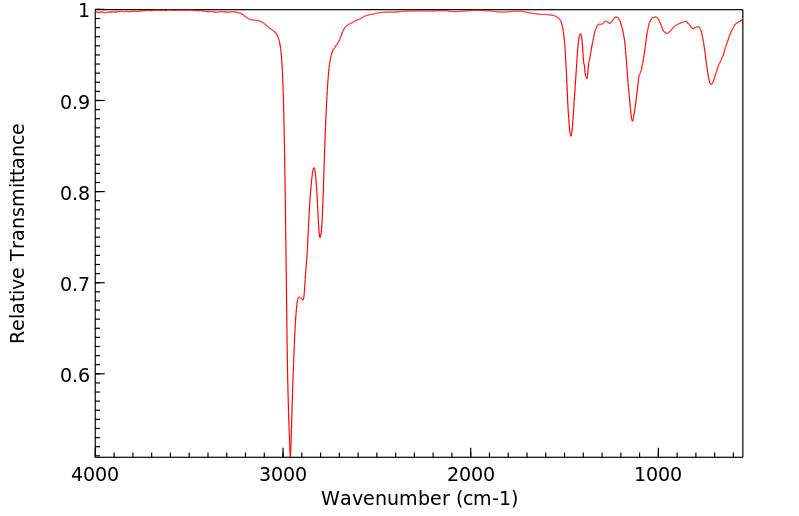
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷