(+/-)-2,3-trans-3,4-cis-7,4'-dimethoxyflavan-3,4-diol
中文名称
——
中文别名
——
英文名称
(+/-)-2,3-trans-3,4-cis-7,4'-dimethoxyflavan-3,4-diol
英文别名
2,3-trans-3,4-cis-4',7-dimethoxyflavan-3,4-diol;(+/-)-7,4'-Dimethoxy-2r(H)-flavan-3c,4c-diol;(+/-)-4',7-Dimethoxy-trans-2,3-flavan-cis-3,4-diol;(+/-)-7-Methoxy-2r-(4-methoxy-phenyl)-chroman-3t,4t-diol;(2R,3S,4S)-7-methoxy-2-(4-methoxyphenyl)-3,4-dihydro-2H-chromene-3,4-diol
CAS
——
化学式
C17H18O5
mdl
——
分子量
302.327
InChiKey
DROLZNWFTXNVAI-YESZJQIVSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):1.7
-
重原子数:22
-
可旋转键数:3
-
环数:3.0
-
sp3杂化的碳原子比例:0.29
-
拓扑面积:68.2
-
氢给体数:2
-
氢受体数:5
上下游信息
反应信息
-
作为反应物:参考文献:名称:732.将相对构型分配给2,3-反式-flavan-3,4-二醇的新方法摘要:DOI:10.1039/jr9640003822
-
作为产物:描述:(+/-)-7-methoxy-2-(4-methoxyphenyl)-2H-chromene 在 水 、 二甲基二环氧乙烷 作用下, 以 丙酮 为溶剂, 以90 %的产率得到(+/-)-2,3-trans-3,4-cis-7,4'-dimethoxyflavan-3,4-diol参考文献:名称:Flav-3-ene 的合成和环氧化作为 Flavan-3,4-diols 仿生制备的方法摘要:由于类黄酮表现出大量的药理特性,如抗氧化、抗炎和抗菌活性,因此人们在这些化合物的医学和治疗应用方面做了大量工作。尽管 flavan-3,4-diols 在许多其他类别的类黄酮的合成中起着核心作用,但仅报道了几种制备这些化合物的方法。在本文中,公开了通过 3 步工艺从容易获得的起始材料制备甲氧基取代的黄烷 3,4-二醇的结果,显示出自然取代模式。还表明,如果在二甲基二氧杂环丙烷 (DMDO) 环氧化过程中添加芳香亲核试剂,则 4-芳基取代的类似物可以从相应的 flav-3-ene 获得。DOI:10.1055/s-0041-1738441
文献信息
-
Steynberg, Jan P.; Ferreira, Daneel; Roux, David G., Journal of the Chemical Society. Perkin transactions I, 1987, p. 1705 - 1712作者:Steynberg, Jan P.、Ferreira, Daneel、Roux, David G.DOI:——日期:——
-
Li, Shaoshun; Onda, Masayuki; Kagawa, Hitoshi, Journal of Heterocyclic Chemistry, 1990, vol. 27, # 7, p. 2029 - 2035作者:Li, Shaoshun、Onda, Masayuki、Kagawa, Hitoshi、Kawase, Hiromi、Iguchi, Mieko、Harigaya, YoshihiroDOI:——日期:——
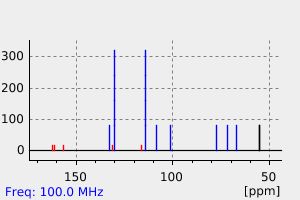
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
([2-(萘-2-基)-4-氧代-4H-色烯-8-基]乙酸)
龙血树脂红血树脂
鼠李素
鼠李柠檬素3-O-beta-D-鼠李三糖苷
鼠李柠檬素
鼠李亭3-O-beta-吡喃葡萄糖苷
黄酮醇-2-磺酸钠盐
黄酮胺
黄酮榕碱
黄酮地洛
黄酮哌酯
黄酮
黄诺马甙
黄苏木素
黄花夹竹桃黄酮
黄芪总皂甙
黄芩黄酮II
黄芩黄酮I
黄芩黄酮
黄芩苷甲酯
黄芩苷
黄芩素磷酸酯
黄芩素一水合物
黄芩素-7-甲醚
黄芩素 6-O-beta-D-吡喃葡萄糖苷
黄芩素
黄烷酮腙
黄烷酮-d5
黄烷酮
黄杞苷
黄宝石羽扇豆素
麗春花青苷
鳞叶甘草素B
高车前苷
高车前素-4'-O-Β-D-葡萄糖苷
高车前素
高良姜素-5-甲基醚
高良姜素-3-甲基醚
高良姜素
高圣草酚-7-O-(6''-O-乙酰基)吡喃葡萄糖苷
高圣草酚
高圣草素-7-O-Β-D-葡萄糖苷
高圣草素
骨碎补素
马里甙
马醉木素
马缨丹黄酮苷
马来酸2-乙酰基-10-[2-(二甲基氨)丙基]-10H-苯并噻嗪正离子
香风草甙
香蒲新苷