三正丁基乙酸锡 | 56-36-0
中文名称
三正丁基乙酸锡
中文别名
乙酸三丁基锡;醋酸三丁锡
英文名称
tributyltin acetate
英文别名
Tributylzinnacetat;Acetoxy-tributyl-stannan;Acetoxy-tributyl-zinn;Tri-n-butyl-zinnacetat;Acetoxy(tri-n-butyl)tin;tributylstannanylium;acetate
CAS
56-36-0
化学式
C14H30O2Sn
mdl
——
分子量
349.101
InChiKey
PWBHRVGYSMBMIO-UHFFFAOYSA-M
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:80-83 °C(lit.)
-
沸点:120 °C / 2mmHg
-
密度:1,27 g/cm3
-
闪点:120 °C
-
溶解度:0.065克/升
-
暴露限值:ACGIH: TWA 0.1 mg/m3; STEL 0.2 mg/m3 (Skin)NIOSH: IDLH 25 mg/m3; TWA 0.1 mg/m3
计算性质
-
辛醇/水分配系数(LogP):4.9
-
重原子数:17
-
可旋转键数:11
-
环数:0.0
-
sp3杂化的碳原子比例:0.93
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
TSCA:No
-
危险等级:6.1
-
危险品标志:T,N
-
安全说明:S35,S36/37/39,S45,S60,S61
-
危险类别码:R48/23/25,R36/38,R21,R50/53,R25
-
WGK Germany:3
-
RTECS号:WH5775000
-
包装等级:III
-
危险类别:6.1
-
危险品运输编号:UN 3146 6.1/PG 2
-
储存条件:库房应保持低温、通风和干燥,并与食品原料分开存放。
SDS
Section 1: Product Identification
Chemical Name: Tri-n-butyltin acetate, 98%
CAS Registry Number: 56-36-0
Formula: (C4H9)3SnOOCCH3
EINECS Number: 200-269-6
Chemical Family: organotin compound
Synonym: Acetoxytributylstanne, Tributylstannyl acetate, Tri-n-butyltin acetate
Section 2: Composition and Information on Ingredients
Ingredient CAS Number Percent ACGIH (TWA) OSHA (PEL)
Title Compound 56-36-0 100% 0.1mg/m3 0.1mg/m3
Section 3: Hazards Identification
Organo tin compounds may act as delayed poisons, causing headache, dizziness; psycho-neurologic
Emergency Overview:
disturbances; sore throat, vomiting; urine retention; vision impairment; skin burns, liver and kidney damage.
Primary Routes of Exposure: Ingestion, inhalation, skin, eyes
Eye Contact: Causes irritation of the eyes.
Skin Contact: Causes irritation of the skin. Prolonged contact may dry the skin and lead to rashes or more severe irritation.
Irritating to the nose, mucous membranes and respiratory tract. High doses may cause long term neurological
Inhalation:
damage.
Delayed and permanent psycho-neurologic disturbances; impairment of vision, unsteadiness, nausea and
Ingestion:
vomiting. Toxic if swallowed.
Irritating to skin, eyes and mucous membranes. Ingestion of certain organotin compounds may cause delayed
Acute Health Affects:
poisoning (4 days) with cerebral edema causing damage to the central nervous system.
Repeated exposure to certain organic tin compounds may cause problems with vision, skin, respiratory
Chronic Health Affects:
system, central nervous system, liver, kidneys, urinary tract, and blood.
NTP: No
IARC: No
OSHA: No
SECTION 4: First Aid Measures
Immediately flush the eyes with copious amounts of water for at least 10-15 minutes. A victim may need
Eye Exposure:
assistance in keeping their eye lids open. Get immediate medical attention.
Wash the affected area with water. Remove contaminated clothes if necessary. Seek medical assistance if
Skin Exposure:
irritation persists.
Remove the victim to fresh air. Closely monitor the victim for signs of respiratory problems, such as difficulty
Inhalation:
in breathing, coughing, wheezing, or pain. In such cases seek immediate medical assistance.
Seek medical attention immediately. Keep the victim calm. Give the victim water (only if conscious). Induce
Ingestion:
vomiting only if directed by medical personnel.
SECTION 5: Fire Fighting Measures
Flash Point: no data
Autoignition Temperature: no data
Explosion Limits: no data
Extinguishing Medium: carbon dioxide or dry powder
Fire fighters should be equipped with a NIOSH approved positive pressure self-contained breathing apparatus
Special Fire Fighting Procedures:
and full protective clothing.
Hazardous Combustion and carbon monoxide, carbon dioxide, soot, organic fumes and tin compounds.
Decomposion Products:
Unusual Fire or Explosion Hazards: No unusual fire or explosion hazards.
SECTION 6: Accidental Release Measures
Small spills can be mixed with vermiculite, ground limestone, sodium carbonate or other suitable
Spill and Leak Procedures:
noncombustible adsorbent and swept up.
SECTION 7: Handling and Storage
Handling and Storage: Store in a cool, dry, well ventilated area away from heat and direct sunlight. Keep containers tightly sealed.
SECTION 8: Exposure Controls and Personal Protection
Eye Protection: Always wear approved safety glasses when handling a chemical substance in the laboratory.
Skin Protection: Wear protective clothing and gloves. Consult with glove manufacturer to determine the proper type of glove.
Ventilation: Work with this product in a well-ventilated area, preferably a fume hood.
If ventilation is not available a respirator should be worn. The use of respirators requires a Respirator
Respirator:
Protection Program to be in compliance with 29 CFR 1910.134.
Ventilation: Work with this product in a well-ventilated area, preferably a fume hood.
Additional Protection: No additional protection required.
SECTION 9: Physical and Chemical Properties
Color and Form: white pwdr.
Molecular Weight: 349.08
Melting Point: 87°
Boiling Point: no data
Vapor Pressure: no data
Specific Gravity: 1.27
Odor: not determined
Solubility in Water: insoluble
SECTION 10: Stability and Reactivity
Stability: moisture sensitive
Hazardous Polymerization: no hazardous polymerization
Conditions to Avoid: none
Incompatibility: strong oxidizing agents and halogens
Decomposition Products: carbon monoxide, carbon dioxide, tin oxide, organic fumes.
SECTION 11: Toxicological Information
Oral (rat); LD50: 99 mg/kg. Intraperitoneal (rat); LDLo: 10 mg/kg. Oral (mouse); LD50: 46 mg/kg. Intravenous
(mouse); LD50: 180 mg/kg. Oral (rabbit); LDLo: 40 mg/kg. Oral (guinea pig); LDLo: 20 mg/kg. Administration
RTECS Data: onto the skin (mammal-species unspecified); LD50: >5 gm/kg. Unreported (mammal-species unspecified);
LD50: 500 mg/kg. Oral (rat); TDLo: 630 mg/kg/12W-C. Oral (rat); TDLo: 176 mg/kg. Oral (rat); TDLo: 176
mg/kg.
Carcinogenic Effects: No data available
Mutagenic Effects: No data available
Tetratogenic Effects: Possible reproductive effector
SECTION 12: Ecological Information
Ecological Information: No information available
SECTION 13: Disposal Considerations
Disposal: Dispose of according to federal, state, and local regulations.
SECTION 14: Transportation
Shipping Name (CFR): Organotin compounds, solid, N.O.S.
Hazard Class (CFR): 6.1
Additional Hazard Class (CFR): NA
Packaging Group (CFR): III
UN ID Number (CFR): UN# 3146
Shipping Name (IATA): Organotin compound, solid, N.O.S.
Hazard Class (IATA): 6.1
Additional Hazard Class (IATA): NA
Packaging Group (IATA): III
UN ID Number (IATA): UN# 3146
SECTION 15: Regulatory Information
TSCA: Not listed in the TSCA inventory.
SARA (Title 313): Title compound not listed.
Second Ingredient: none
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
上下游信息
反应信息
-
作为反应物:参考文献:名称:Gmelin Handbuch der Anorganischen Chemie, Gmelin Handbook: Sn: Org.Verb.2, 1.1.2.5.1, page 272 - 276摘要:DOI:
-
作为产物:参考文献:名称:某些含有SnO键的有机锡(IV)化合物的合成和光谱研究摘要:描述了许多含有Sn = O键的有机锡(IV)化合物的制备。介绍了它们的13 C和1 H NMR,IR光谱以及一些分子量的测定结果。假定结构主要基于一个键的耦合常数。从1 H NMR和IR光谱获得进一步的证据。对于所报告的化合物,由于在分配和解释CO,SnO和SnC拉伸频率方面存在困难,IR光谱似乎没有提供明确的证据。DOI:10.1016/0022-1902(79)80173-6
-
作为试剂:描述:(2S,5S)-5-(5-acetoxy-2,2-dimethyl-4,6-dioxo-1,3-dioxan-5-yl)-2,5-dihydrofuran-2-yl benzoate 、 6-氯嘌呤 在 tris(dibenzylideneacetone)dipalladium(0) chloroform complex 、 三乙胺 、 三正丁基乙酸锡 、 (1R,2R)-(+)-1,2-二氨基环己烷-N,N′-双(2-二苯基磷苯甲酰) 作用下, 以 四氢呋喃 为溶剂, 反应 24.0h, 以83%的产率得到5-((2S,5R)-5-(6-chloro-9H-purin-9-yl)-2,5-dihydrofuran-2-yl)-2,2-dimethyl-4,6-dioxo-1,3-dioxan-5-yl acetate参考文献:名称:Acetoxy Meldrum 的酸:Pd 催化的不对称烯丙基烷基化中的多功能酰基阴离子等价物摘要:Acetoxy Meldrum 的酸可以在 Pd 催化的不对称烯丙基烷基化中用作通用的酰基阴离子等价物。该亲核试剂与各种内消旋和外消旋亲电试剂的反应以高产率和对映纯度提供烷基化产物。这些对映体富集的产品是多功能中间体,可以使用以氮和氧为中心的亲核试剂进一步功能化,为合成核苷类似物提供多功能支架。这些支架用于完成抗 HIV 药物卡波韦、阿巴卡韦和抗生素阿里斯特霉素的正式合成。DOI:10.1021/ol2011242
文献信息
-
Novel Carbon-Carbon Bond Formation through Mizoroki-Heck Type Reaction of Silanols and Organotin Compounds作者:Kazunori Hirabayashi、Jun-ichi Ando、Jun Kawashima、Yasushi Nishihara、Atsunori Mori、Tamejiro HiyamaDOI:10.1246/bcsj.73.1409日期:2000.6The reaction of dimethyl(phenyl)silanol with butyl acrylate in the presence of a stoichiometric amount of Pd(OAc)2 or by a combined use of 0.1 molar amount of Pd(OAc)2 and Cu(OAc)2/LiOAc (molar ratio 3/2) gave butyl cinnamate in 76% or 57% yield, respectively. The similar reaction with tributyl(phenyl)tin also proceeded in 77% yield. The organotin compound was shown to react faster than the silanol
-
INHIBITORS OF PLASMA KALLIKREIN AND USES THEREOF申请人:Shire Human Genetic Therapies, Inc.公开号:US20190284182A1公开(公告)日:2019-09-19The present invention provides compounds and compositions thereof which are useful as inhibitors of plasma kallikrein and which exhibit desirable characteristics for the same.本发明提供了作为血浆激肽酶抑制剂并具有相同理想特性的化合物和组合物。
-
The mechanism of reaction of tetraalkyltins with mercuric carboxylates in methanol作者:M.H. Abraham、Davood Farshbaf Dadjour、C.J. HollowayDOI:10.1016/s0022-328x(00)95129-5日期:1973.5In the reaction of mercuric carboxylates with tetraalkyltins in methanol, rate constrants for attack of the species (RCO2)2 Hg increase along the series R = t-Bu < Et < Me < Ph < ClCH2CH2 < MeOCH2 < ClCH2 which suggests an open transition state for these SE2 substitutions.
-
Organometallic reactions. Part VI. The addition of the Sn–O bond to the carbonyl group作者:Alwyn G. Davies、W. R. SymesDOI:10.1039/j39670001009日期:——with other carbonyl compounds by a reversible exchange of the organotin reagent. Acetyl chloride or methanol similarly react by displacing the carbonyl compound OCR″R‴. If one or both of the groups R″ and R‴ is a trihalogenomethyl group, methanolysis can alternatively involve displacement of the carbonyl compound R″CO·OR′, liberating the haloform; in consequence, organotin compounds catalyse the methanolysis甲醇三丁基锡和双三丁基锡氧化物在许多醛和某些更具反应性的酮的羰基上反应,生成1:1加合物(A)。在氯醛和1,1,3-三氯-1,3,3-三氟丙烷-2-一的情况下,核磁共振谱表明还可以形成1:2加合物(B),并与氯醛重复生成通过该过程,得到聚合物(C)。R 3 Sn·OR [图形省略] R 3 Sn·O·CR″ R″′·OR′图形(A)R 3 Sn·[O·CR″ R″′] 2 ·OR′[图形省略] R 3 Sn·[O·CR″ R″′] a·OR'(B)(C)通过有机锡试剂的可逆交换,1:1加合物(A)可以与其他羰基化合物反应。类似地,乙酰氯或甲醇通过取代羰基化合物OCR” R 4进行反应。如果基团R”和R 4中的一个或两个是三卤代甲基,则甲醇分解可以替代地涉及羰基化合物R” CO·OR'的置换,从而释放出卤代形式。结果,有机锡化合物催化六氯丙酮的甲醇分解为三氯乙酸甲酯和氯仿。建议所有这些反应通过一个六元循环过渡态进行。
-
Hydrostannolysis reactions—II作者:L.E. Khoo、H.H. LeeDOI:10.1016/s0040-4020(01)93069-8日期:1970.1Reaction of phenyl benzoate with tri-n-butyltin hydride has been found to give mainly toluene, benzyl benzoate, tri-n-butyltin phenoxide and tri-n-butyltin benzoate. Possible reaction paths leading to these products are discussed. A SH2 mechanism involving initial attack of tri-n-butyltin radical at the ethereal oxygen atom of the ester with displacement of the benzoyl moiety appears to be consistent
表征谱图
-
氢谱1HNMR
-
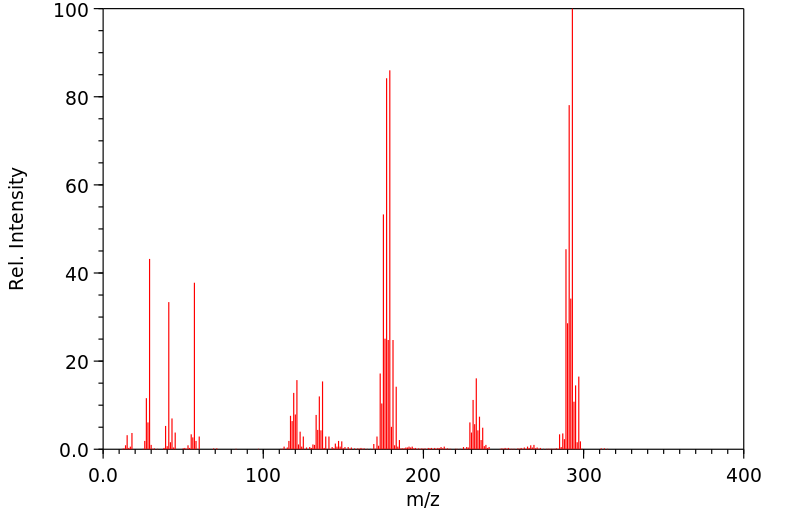
质谱MS
-
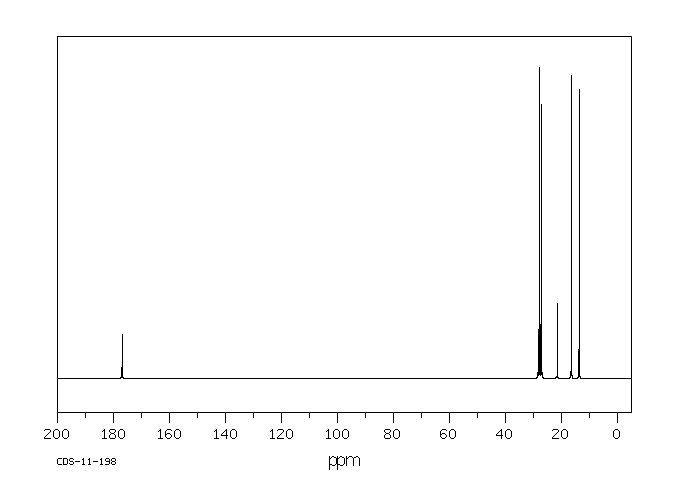
碳谱13CNMR
-
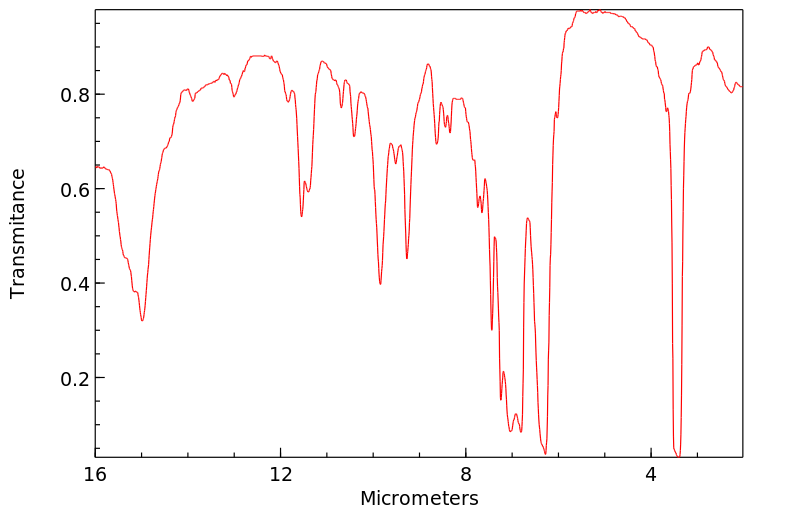
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
锰,五羰基(三甲基甲锡烷基)-,(OC-6-22)-
锡烷,乙氧基三甲基-
锡烷,三丁基(1E)-1-庚烯基-
锡烷,三丁基(1-甲基-2-丁烯基)-,(Z)-
锡烷,三(1,1-二甲基乙基)乙炔基-
锡烷,(4-氯二环[2.2.1]庚-1-基)三甲基-
锡烷,(1E)-1-丁烯-3-炔基三丁基-
铝,三庚基-
铝,丁氧基二(2-甲基丙基)-
铅烷,三丁基-1-己炔基-
辛基锡
辛基氧代锡烷
膦,三(三甲基甲锡烷基)-
碳化铝
碘化三乙基铅
碘(三甲基)铅烷
硼烷胺,N,N-二(氯二甲基甲锡烷基)-1,1-二甲基-
硫烷负离子三甲基铅
硫代乙酸 S-[3-(三丁基锡烷基)丙基]酯
硒基二(三甲基锡)
癸酰(二羟基)铝
甲硫基三丁基锡烷
甲烷四基四(三甲基锡烷)
甲氧基二(2-甲基丙基)-铝
甲基锡
甲基烯丙基三正丁基锡
甲基氢化钼
甲基双(1-甲基环己基)锡烷
甲基二氯化铝
甲基三戊基锡
甲基(三丙基)锡烷
环己羧酸,2-氨基-,甲基酯,(1S,2S)-
环己基三异丙基锡烷
环己基[(三丁基锡烷基)氧基]重氮1-氧化物
环己基-三甲基锡烷
环己基(异丙基)二甲基锡烷
环丙基(三异丙基)锡烷
烯丙基三甲基锡烷
烯丙基三乙烯基锡烷
烯丙基三丁基锡
烯丙基三(3,3,4,4,5,5,6,6,7,7,8,8,8-十三氟辛基)锡烷
溴二乙基铝
溴三甲基铅
溴(异丙基)汞
溴(三乙基)铅
溴(三丁基)铅
氰酸三丁基锡烷
氯甲氧基甲基三丁基锡
氯甲基三甲基锡
氯化二己基铝