代谢
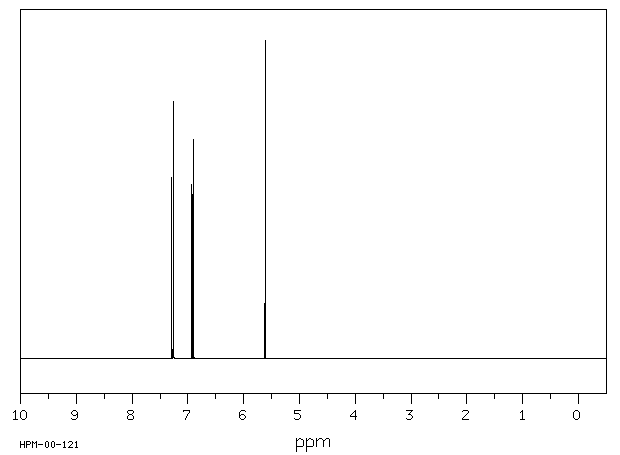
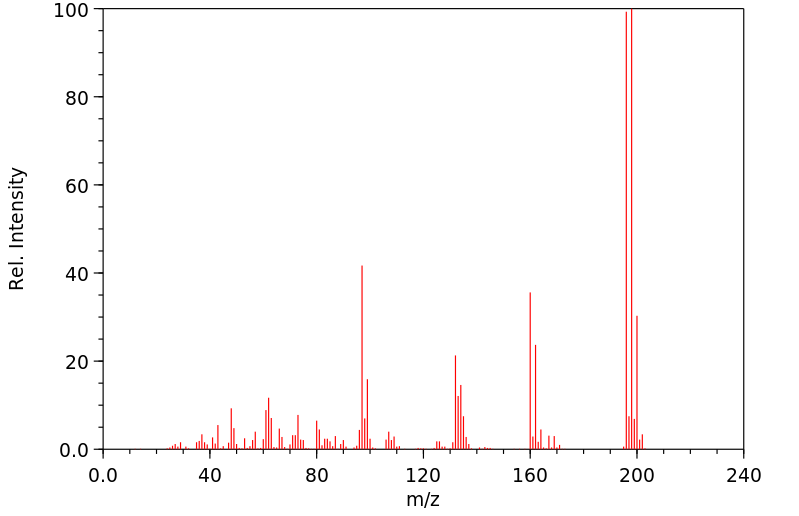
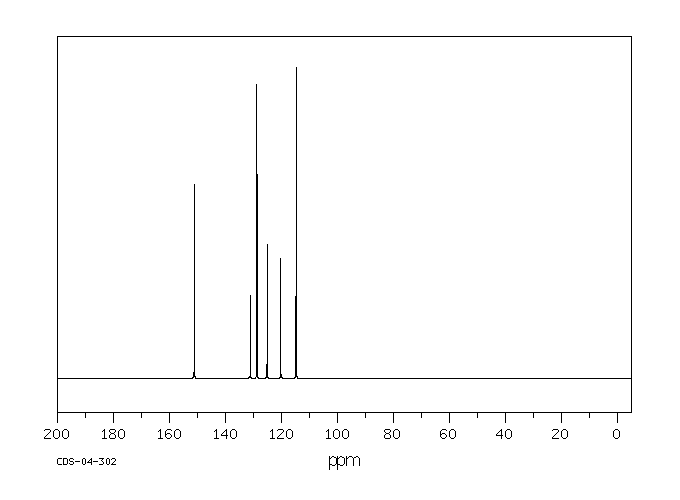
老鼠将α-六氯环己烷代谢为1,2,4-三氯苯、2,3,4-三氯酚、2,4,6-三氯酚、其他三氯酚以及2,3,4,6-四氯酚。这些物质通过尿液排出。α-六氯环己烷还转化为3,4,6/5-五氯环己烯,这种物质在肾脏中可被检测到,但在尿液中检测不到。
Rats metabolized alpha-hexachlorocyclohexane to 1,2,4-trichlorobenzene, 2,3,4-trichlorophenol, 2,4,6-trichlorophenol, other trichlorophenols, and 2,3,4,6-tetrachlorophenol. These were excreted in urine. Alpha-hexachlorocyclohexane is also converted to 3,4,6/5-pentachlorocyclohexene which is detectable in kidneys, but not in urine.
来源:Hazardous Substances Data Bank (HSDB)