三辛基硅烷 | 18765-09-8
中文名称
三辛基硅烷
中文别名
溴甲基-1,3-二恶烷;2-(2-溴乙基)-1,3-二噁茂烷;三正辛基一氢硅烷;溴甲基-1,3-二烷;2-(2-溴乙基)-1,3-二氧环戊烷;2-(2-溴乙基)-1,3-二噁烷;2-(2-溴乙基)-1,3-二恶茂烷;2-(2-溴乙基)-1,3-二氧戊环
英文名称
tri-n-octylsilane
英文别名
tri(n-octyl)silane;trioctylsilane;Oct3SiH;(n-Oct)3SiH;Tri-octyl-silan;Trioctylsilane, 95%
CAS
18765-09-8
化学式
C24H52Si
mdl
——
分子量
368.762
InChiKey
JBAALNCKQCMFDH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:<0°C
-
沸点:163-165 °C/0.15 mmHg (lit.)
-
密度:0.821 g/mL at 25 °C (lit.)
-
闪点:>230 °F
-
稳定性/保质期:
按规定使用不会分解,避免与氧化剂、酸或碱接触。
计算性质
-
辛醇/水分配系数(LogP):9.29
-
重原子数:25
-
可旋转键数:21
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
TSCA:Yes
-
危险品标志:Xi
-
安全说明:S26,S36
-
危险类别码:R36/37/38
-
WGK Germany:1
-
危险标志:GHS07
-
危险性描述:H315,H319
-
危险性防范说明:P305 + P351 + P338
SDS
模块 1. 化学品
1.1 产品标识符
: 三辛基硅烷
产品名称
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅用于研发。不作为药品、家庭或其它用途。
模块 2. 危险性概述
2.1 GHS-分类
皮肤刺激 (类别 2)
眼睛刺激 (类别 2A)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 警告
危险申明
H315 造成皮肤刺激。
H319 造成严重眼刺激。
警告申明
预防
P264 操作后彻底清洁皮肤。
P280 穿戴防护手套/ 眼保护罩/ 面部保护罩。
响应
P302 + P352 如皮肤接触:用大量肥皂和水清洗。
P305 + P351 + P338 如与眼睛接触,用水缓慢温和地冲洗几分钟。如戴隐形眼镜并可方便地取
出,取出隐形眼镜,然后继续冲洗.
P321 具体治疗(见本标签上提供的急救指导)。
P332 + P313 如发生皮肤刺激:求医/ 就诊。
P337 + P313 如仍觉眼睛刺激:求医/就诊。
P362 脱掉沾染的衣服,清洗后方可重新使用。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C24H52Si
分子式
: 368.76 g/mol
分子量
组分 浓度或浓度范围
Trioctylsilane
-
化学文摘登记号(CAS 18765-09-8
No.) 242-559-5
EC-编号
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 向到现场的医生出示此安全技术说明书。
吸入
如果吸入,请将患者移到新鲜空气处。 如呼吸停止,进行人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用大量水彻底冲洗至少15分钟并请教医生。
食入
切勿给失去知觉者通过口喂任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,抗乙醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 二氧化硅
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 作业人员防护措施、防护装备和应急处置程序
使用个人防护用品。 避免吸入蒸气、烟雾或气体。 保证充分的通风。
6.2 环境保护措施
不要让产品进入下水道。
6.3 泄漏化学品的收容、清除方法及所使用的处置材料
用惰性吸附材料吸收并当作危险废物处理。 放入合适的封闭的容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 避免吸入蒸气和烟雾。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 使容器保持密闭,储存在干燥通风处。
打开了的容器必须仔细重新封口并保持竖放位置以防止泄漏。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
根据良好的工业卫生和安全规范进行操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
带有防护边罩的安全眼镜符合 EN166要求请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟)
检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
防渗透的衣服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和数量来选择。
呼吸系统防护
如危险性评测显示需要使用空气净化的防毒面具,请使用全面罩式多功能防毒面具(US)或ABEK型
(EN
14387)防毒面具筒作为工程控制的候补。如果防毒面具是保护的唯一方式,则使用全面罩式送风防
毒面具。 呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 液体
颜色: 无色
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
无数据资料
f) 沸点、初沸点和沸程
163 - 165 °C 在 0.20 hPa - lit.
g) 闪点
113 °C - 闭杯
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 蒸汽密度
无数据资料
m) 密度/相对密度
0.821 g/cm3 在 25 °C
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应
无数据资料
10.4 应避免的条件
无数据资料
10.5 不相容的物质
强氧化剂强氧化剂, 酸, 碱
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 通过皮肤吸收可能有害。 造成皮肤刺激。
眼睛 造成严重眼刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久性和降解性
无数据资料
12.3 潜在的生物累积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不良影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和不可回收的溶液交给有许可证的公司处理。
受污染的容器和包装
按未用产品处置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国运输名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 国际空运危规: 否
海洋污染物(是/否): 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
上下游信息
反应信息
-
作为反应物:描述:三辛基硅烷 在 C15H27Br2CoN3 、 potassium tert-butylate 、 水 作用下, 以 1,4-二氧六环 为溶剂, 反应 2.0h, 以86%的产率得到hexaoctyl-disiloxane参考文献:名称:钴催化的二硅氧烷和氢二硅氧烷的选择性合成摘要:分别从硅烷和二氢硅烷与水的反应中获得对称的硅氧烷和环四硅氧烷的选择性合成,并且该反应由NNN H t Bu钴(II)钳形配合物催化。有趣的是,当苯硅烷用水进行催化时,获得了由12个硅和18个氧中心组成的硅氧烷笼,反应明显进行,释放出3当量的分子氢(36 H 2)在温和的实验条件下。在硅烷与不同的硅烷醇反应后,通过钴催化实现了高选择性和受控的高级单氢硅氧烷和二甲氧基单氢硅烷的合成。释放的分子氢是在所有这些转化中观察到的唯一副产物。机理研究表明,反应是通过均质途径发生的。动力学和独立实验证实了硅烷催化氧化为硅烷醇的过程,并且进一步的脱氢偶联过程还涉及对称硅氧烷,环四硅氧烷和硅氧烷笼型化合物的合成,而硅烷和硅烷醇的不对称一氢硅氧烷则通过脱氢偶联反应进行合成。总体而言,这些钴催化的氧化偶联反应基于Si–H,Si–OH,和硅烷,硅烷醇和水的O–H键活化。建议使用由Co(II)中间体组成的催化循环。DOI:10.1021/acscatal.9b00305
-
作为产物:参考文献:名称:应用于液相肽合成的硅基疏水标签:受保护的DRGN-1和聚丙氨酸链合成摘要:开发了两种新的可回收硅基疏水标签,用于将它们安装在肽的 C 端,以增加在有机溶剂中的溶解度和液相肽合成 (LPPS) 过程中肽的反应性。它们包含含有甲硅烷氧基的标签和含有芳基甲硅烷基的标签。这些标签的疏水性比以前报道的例子要大得多。含有甲硅烷氧基的标签与Fmoc-脱保护条件(Fmoc-化学)和氢化条件(Cbz-化学)相容,而含有芳基甲硅烷基的标记对Fmoc-脱保护条件(Fmoc-化学)和Boc-脱保护条件具有抗性( Boc-化学)。使用含甲硅烷氧基的标签,受保护的 DRGN-1,采用线性合成结合一个收敛合成步骤成功制备了含有14个氨基酸残基的多肽。使用含有芳基甲硅烷基的标签,合成了含有 7 个丙氨酸残基的聚丙氨酸链。含有芳基甲硅烷基的标签也可以安装在 N 端,以将肽从 N 端延伸到 C 端。在这些硅基标签的帮助下,每一步的产率和产品在有机溶剂中的溶解度都非常好。DOI:10.1039/d2ob01795d
-
作为试剂:描述:endo-bicyclo[2.2.1]hept-5-ene-2,3-dicarboxylic dimethyl ester 、 1-(4-acetylphenyl)diazonium tetrafluoroborate 在 三辛基硅烷 、 palladium diacetate 作用下, 以 四氢呋喃 为溶剂, 反应 1.5h, 以58%的产率得到endo-cis-2,3-dimethoxycarbonylnorbornane参考文献:名称:Aryl Norbornanes and Analogues via Palladium-Catalyzed Hydroarylation with Arenediazonium Tetrafluoroborates摘要:钯催化的芳香重氮四氟硼酸盐与降冰片烯衍生物及其类似物在Pd(OAc)2和i-Pr3SiH存在下于THF中的氢芳基化反应,可以高至很好的产率得到在exo位置含有添加芳基单元的水合芳基产物。该反应容忍多种有用的官能团,并可以采用一锅法进行,即现场生成芳香重氮盐。DOI:10.1055/s-2008-1078051
文献信息
-
Olefin hydrosilylation catalyzed by cationic nickel(<scp>ii</scp>) allyl complexes: a non-innocent allyl ligand-assisted mechanism作者:J. Mathew、Y. Nakajima、Y.-K. Choe、Y. Urabe、W. Ando、K. Sato、S. ShimadaDOI:10.1039/c6cc01665k日期:——The arene-supported cationic nickel allyl complexes serve as good catalysts for olefin hydrosilylation at room temperature. Detailed mechanistic studies based on experiments and DFT calculations support the novel mechanism, which...
-
SATURATED AND UNSATURATED SILAHYDROCARBONS VIA IRON AND COBALT PYRIDINE DIIMINE CATALYZED OLEFIN SILYLATION申请人:Lewis Kenrick Martin公开号:US20140330036A1公开(公告)日:2014-11-06The present invention relates to processes for the synthesis of saturated and unsaturated silahydrocarbons using iron-containing or cobalt-containing catalysts. The processes of the invention can produce tetraalkylsilanes, phenyltrialkylsilanes, substituted phenyltrialkylsilanes and their mixtures, which are useful as lubricants and hydraulic fluids, as well as alkyl alkenylsilanes, phenyl alkenylsilanes and substituted phenyl alkenylsilanes and their mixtures, which are useful in the synthesis of saturated silahydrocarbons and other organofunctional silanes.
-
Synthesis of β-Silyl α-Amino Acids via Visible-Light-Mediated Hydrosilylation作者:Yi Wan、Jiajie Zhu、Qiyang Yuan、Wei Wang、Yongqiang ZhangDOI:10.1021/acs.orglett.1c00065日期:2021.2.19of β-silyl α-amino acids is reported via the application of visible-light-mediated hydrosilylation. The reaction utilizes readily accessible and structurally diverse hydrosilanes to provide radicals for conjugate addition to dehydroalanine ester and analogues. Notably, the use of chiral methyleneoxazolidinone as the substrate and chiral inducer enabled the highly stereoselective synthesis. Furthermore
-
Regioselective Hydrosilylation of Olefins Catalyzed by a Molecular Calcium Hydride Cation作者:Danny Schuhknecht、Thomas P. Spaniol、Laurent Maron、Jun OkudaDOI:10.1002/anie.201909585日期:2020.1.2Chemo- and regioselectivity are often difficult to control during olefin hydrosilylation catalyzed by d- and f-block metal complexes. The cationic hydride of calcium [CaH]+ stabilized by an NNNN macrocycle was found to catalyze the regioselective hydrosilylation of aliphatic olefins to give anti-Markovnikov products, while aryl-substituted olefins were hydrosilyated with Markovnikov regioselectivity
-
Oxyfunctionalization reactions by perfluoro cis-2,3-dialkyloxaziridines. Enantioselective conversion of silanes into silanols作者:Marcello Cavicchioli、Vittorio Montanari、Giuseppe ResnatiDOI:10.1016/s0040-4039(00)73424-1日期:1994.8Perfluoro cis-2,3-dialkyloxaziridine 2 is shown to perform the oxyfunctionalization of silanes 1 under very mild conditions to give silanols and silanediols 3 in high chemical yields and complete enantioselectivity.
表征谱图
-
氢谱1HNMR
-
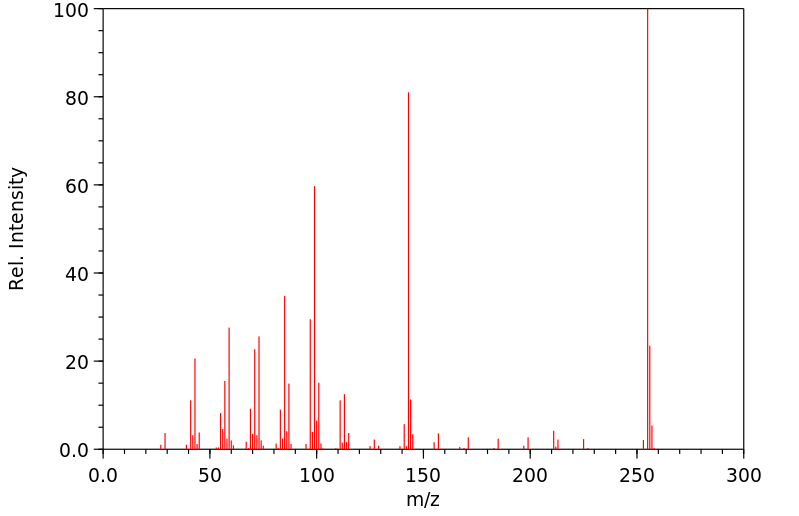
质谱MS
-
碳谱13CNMR
-
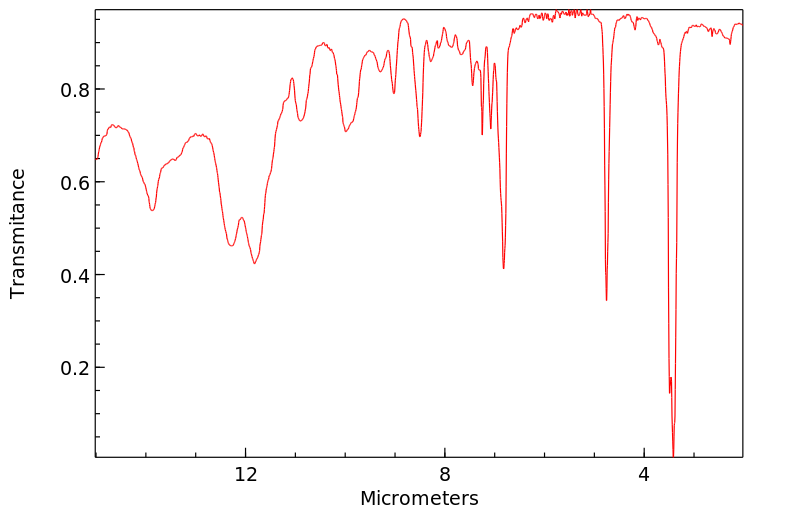
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
镁己烷
锌,二环己基-
锂,3-辛炔基-
锂,(1-苯基乙基)-
铜(I)己基乙炔化物
铜(1+),2-甲基丙烷
铅杂鎓,二乙基甲基-
钠,(1,2,3,4-四甲基-2,4-环戊二烯-1-基)-
钛(4+)四(2,2-二甲基丙烷-1-I去)
邻苯二甲酰基
邻甲基二苯甲酮自由基阳离子
辛烷钠
苄基铜
苄基钠
脱羰秋水仙碱
胂,二(2,2-二甲基丙基)-
纳米碳化钛
红陪酚四甲基醚
红倍酚
秋水仙碱甲硫代磺酸盐
秋水仙碱
碳化锆
碳化铪
碳化铌
碳化铀
碳化钽
碳化钒
碘二氟甲基(1+)
硼化二铬
硫代秋水仙碱
硅烷,二甲基丙基-
硅烷,乙基二甲基-2-丙烯基-
硅烷,乙基二(3-甲基丁基)-
石墨溴化物
甲烷,钼
甲基锡烷
甲基铍氢化物
甲基辛基硅烷
甲基硅烷基阳离子
甲基硅烷
甲基二乙烯基硅烷
甲基丙烯酸7-氧代-4-(苯基偶氮)-1,3,5-环庚三烯-1-基酯
甲基三烯丙基硅烷
甲基三正辛基硅烷
甲基三正己基硅烷
甲基三乙基硅烷
甲基三-N-癸基硅烷
甲基6-肼基-7-氧代-1,3,5-环庚三烯-1-羧酸酯
甲基-三-n-丁基硅烷
甲基(三丙基)硅烷