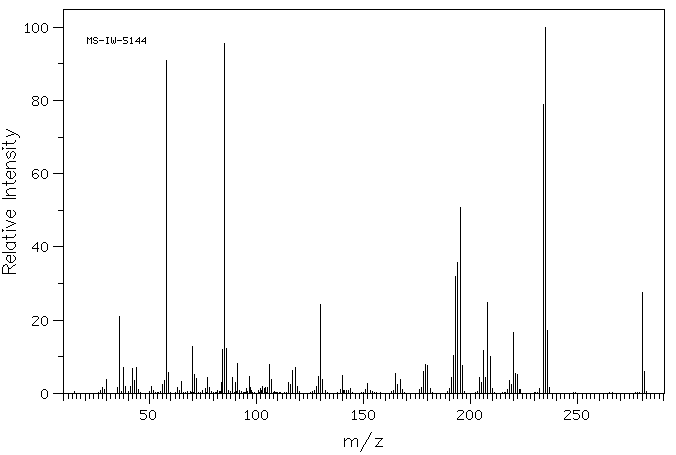
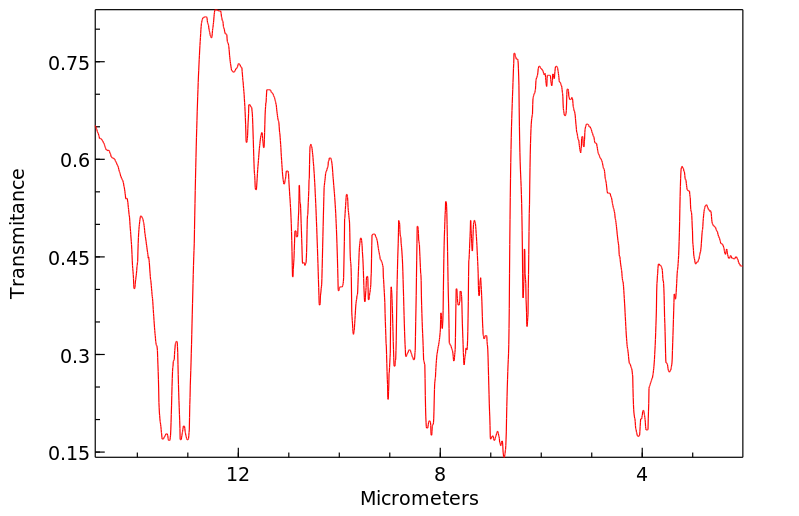
毒理性
◉ 母乳喂养期间使用总结:丙咪嗪及其代谢物在母乳中的水平较低,在哺乳婴儿的血清中尚未检测到。未报告立即副作用,有限的随访发现对婴儿的生长和发育没有不良影响。在母乳喂养期间使用丙咪嗪通常不会预期对哺乳婴儿产生任何不良影响,尤其是如果婴儿超过2个月大。一个安全评分系统认为在哺乳期间使用丙咪嗪是可能的。一些专家认为丙咪嗪是哺乳母亲首选的抗抑郁药之一。然而,当需要大剂量或在哺乳新生儿或早产儿时,可能更倾向于使用其他药物。
◉ 对哺乳婴儿的影响:在一项研究中,一名母亲在睡前连续15天每天服用200毫克丙咪嗪的情况下,她6周大的哺乳婴儿没有出现行为或身体变化。
在另一项研究中,14名哺乳婴儿的母亲平均每天服用161毫克丙咪嗪(范围125至225毫克/天),对这些婴儿进行了1到3年的随访,发现对他们的生长和发育没有不良影响。
在另一项研究中,4名婴儿在母亲每天服用75至150毫克丙咪嗪的情况下,分别于产后2周(3名婴儿)和8周(1名婴儿)开始哺乳,持续7至18周。正式测试表明,直到30个月大,婴儿的发育没有受到不良影响。其中一名婴儿的母亲除了服用丙咪嗪150毫克外,还服用了氯丙嗪。
在另一项研究中,25名在孕期和哺乳期间服用三环类抗抑郁药的母亲的婴儿在15至71个月时进行了正式测试,发现他们的生长和发育正常。其中一些母亲服用了丙咪嗪。
六名产后被诊断为恐慌障碍的母亲成功接受了每天25至35毫克(平均28毫克)丙咪嗪的治疗,平均在产后5.9周开始治疗。母亲平均治疗了9.3周。所有母亲报告说他们的婴儿没有出现不良反应。
◉ 对泌乳和母乳的影响:丙咪嗪已在非妊娠、非哺乳患者中引起催乳素水平升高和男性乳房发育。偶有报道出现乳汁分泌过多。这些发现对哺乳母亲的临床意义尚不清楚。已建立泌乳的母亲催乳素水平可能不会影响她的哺乳能力。
在一项观察性研究中,调查了2859名在怀孕前两年内服用过抗抑郁药的妇女的结果。与怀孕期间没有服用抗抑郁药的妇女相比,整个孕期都服用抗抑郁药的妇女在出院时哺乳的可能性降低了37%。仅在第三孕期服用抗抑郁药的妇女在出院时哺乳的可能性降低了75%。仅在第一和第二孕期服用抗抑郁药的妇女在出院时哺乳的可能性没有降低。研究中没有具体说明母亲使用的抗抑郁药种类。
在一项回顾性队列研究中,比较了2001年至2008年医院电子医疗记录中的晚期妊娠期间被开出抗抑郁药的妇女(n = 575)与患有精神疾病但未接受抗抑郁药的妇女(n = 1552)以及没有精神疾病诊断的妇女(n = 30,535)。接受抗抑郁药治疗的妇女在出院时哺乳的可能性比没有精神疾病诊断的妇女低37%,但与未接受治疗的患有精神疾病的母亲相比,哺乳的可能性没有降低。这些母亲中没有人在服用丙咪嗪。
在一项针对1999年至2008年的80,882对挪威母婴对的研究中,有392名妇女报告在产后新使用了抗抑郁药,其中201人报告她们从孕期继续使用抗抑郁药。与未暴露的对照组相比,晚期妊娠使用抗抑郁药与哺乳开始的几率降低7%有关,但对哺乳持续时间或专一性没有影响。与未暴露的对照组相比,新开始或重新开始使用抗抑郁药与在6个月时主要哺乳的几率降低63%,任何哺乳的几率降低51%,以及突然停止哺乳的风险增加2.6倍。具体抗抑郁药没有提及。
◉ Summary of Use during Lactation:Milk levels of imipramine and its metabolite are low and have not been detected in the serum of breastfed infants. Immediate side effects have not been reported and a limited amount of follow-up has found no adverse effects on infant growth and development. Imipramine use during breastfeeding would usually not be expected to cause any adverse effects in breastfed infants, especially if the infant is older than 2 months. A safety scoring system finds imipramine use to be possible during breastfeeding. Some experts consider imipramine one of the antidepressants of choice for nursing mothers. However, other agents may be preferred when large doses are required or while nursing a newborn or preterm infant.
◉ Effects in Breastfed Infants:No behavioral or physical changes were noted in a 6-week-old breastfed infant whose mother had been taking imipramine 200 mg daily at bedtime for 15 days.
Follow-up for 1 to 3 years in 14 breastfed infants whose mothers were taking imipramine in an average dosage of 161 mg daily (range 125 to 225 mg daily) found no adverse effects on growth and development.
Four infants were breastfed for 7 to 18 weeks during maternal use of imipramine 75 to 150 mg daily starting at 2 weeks (3 infants) and 8 weeks (1 infant) postpartum. Formal testing indicated no adverse effects on infant development up to 30 months of age. The mother of 1 infant was taking haloperidol along with imipramine 150 mg daily.
In another study, 25 infants whose mothers took a tricyclic antidepressant during pregnancy and lactation were tested formally between 15 to 71 months and found to have normal growth and development. Some of the mothers were taking imipramine.
Six postpartum mothers diagnosed with panic disorder were successfully treated with imipramine 25 to 35 mg (mean 28 mg) daily starting at a mean of 5.9 weeks postpartum. Mothers were treated for a mean of 9.3 weeks. All mothers reported that no adverse effects occurred in their infants.
◉ Effects on Lactation and Breastmilk:Imipramine has caused increased prolactin levels and gynecomastia in nonpregnant, nonnursing patients. Galactorrhea has been reported rarely. The clinical relevance of these findings in nursing mothers is not known. The prolactin level in a mother with established lactation may not affect her ability to breastfeed.
An observational study looked at outcomes of 2859 women who took an antidepressant during the 2 years prior to pregnancy. Compared to women who did not take an antidepressant during pregnancy, mothers who took an antidepressant during all 3 trimesters of pregnancy were 37% less likely to be breastfeeding upon hospital discharge. Mothers who took an antidepressant only during the third trimester were 75% less likely to be breastfeeding at discharge. Those who took an antidepressant only during the first and second trimesters did not have a reduced likelihood of breastfeeding at discharge. The antidepressants used by the mothers were not specified.
A retrospective cohort study of hospital electronic medical records from 2001 to 2008 compared women who had been dispensed an antidepressant during late gestation (n = 575) to those who had a psychiatric illness but did not receive an antidepressant (n = 1552) and mothers who did not have a psychiatric diagnosis (n = 30,535). Women who received an antidepressant were 37% less likely to be breastfeeding at discharge than women without a psychiatric diagnosis, but no less likely to be breastfeeding than untreated mothers with a psychiatric diagnosis. None of the mothers were taking imipramine.
In a study of 80,882 Norwegian mother-infant pairs from 1999 to 2008, new postpartum antidepressant use was reported by 392 women and 201 reported that they continued antidepressants from pregnancy. Compared with the unexposed comparison group, late pregnancy antidepressant use was associated with a 7% reduced likelihood of breastfeeding initiation, but with no effect on breastfeeding duration or exclusivity. Compared with the unexposed comparison group, new or restarted antidepressant use was associated with a 63% reduced likelihood of predominant, and a 51% reduced likelihood of any breastfeeding at 6 months, as well as a 2.6-fold increased risk of abrupt breastfeeding discontinuation. Specific antidepressants were not mentioned.
来源:Drugs and Lactation Database (LactMed)