二乙基(苯基硫代甲基)磷酸酯 | 38066-16-9
中文名称
二乙基(苯基硫代甲基)磷酸酯
中文别名
苯硫甲基磷酸二乙酯;苯硫甲基膦酸二乙酯
英文名称
diethyl [(phenylthio)methyl]phosphonate
英文别名
diethyl [(phenylsulfanyl)methyl]phosphonate;Diethyl phenylthiomethylphosphonate;diethoxyphosphorylmethylsulfanylbenzene
CAS
38066-16-9
化学式
C11H17O3PS
mdl
MFCD00015130
分子量
260.294
InChiKey
FBUXEPJJFVDUFE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:146-148 °C (0.5 mmHg)
-
密度:1.17
-
闪点:146-148°C/0.5mm
-
溶解度:难溶于水
-
稳定性/保质期:
常规情况下不会分解,也没有任何危险反应。
计算性质
-
辛醇/水分配系数(LogP):2.5
-
重原子数:16
-
可旋转键数:7
-
环数:1.0
-
sp3杂化的碳原子比例:0.454
-
拓扑面积:60.8
-
氢给体数:0
-
氢受体数:4
安全信息
-
危险品标志:Xi
-
危险类别码:R36/37/38
-
海关编码:2931900090
-
安全说明:S26,S37/39
-
储存条件:密封、阴凉、干燥保存。
SDS
| Name: | Diethyl(Phenylthiomethyl)Phosphonate Material Safety Data Sheet |
| Synonym: | None Known |
| CAS: | 38066-16-9 |
Synonym:None Known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 38066-16-9 | Diethyl(Phenylthiomethyl)Phosphonate | ca. 100 | 253-770-7 |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.
Potential Health Effects
Eye:
Causes eye irritation. May cause chemical conjunctivitis.
Skin:
Causes skin irritation.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea. The toxicological properties of this substance have not been fully investigated.
Inhalation:
Causes respiratory tract irritation. The toxicological properties of this substance have not been fully investigated. Can produce delayed pulmonary edema.
Chronic:
Effects may be delayed.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid. Do NOT use mouth-to-mouth resuscitation.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas. Runoff from fire control or dilution water may cause pollution.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Avoid runoff into storm sewers and ditches which lead to waterways. Clean up spills immediately, observing precautions in the Protective Equipment section. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation. Use with adequate ventilation. Wash clothing before reuse.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 38066-16-9: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: colorless
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 146 - 148 deg C @ .50mmHg
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: 1.1740g/cm3
Molecular Formula: C11H17O3PS
Molecular Weight: 260.151
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Incompatible materials, excess heat.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 38066-16-9 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Diethyl(Phenylthiomethyl)Phosphonate - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
IMO
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
RID/ADR
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing group:
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 37/39 Wear suitable gloves and eye/face
protection.
WGK (Water Danger/Protection)
CAS# 38066-16-9: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 38066-16-9 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 38066-16-9 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— diethyl (chloro(phenylthio)methyl)phosphonate 59664-75-4 C11H16ClO3PS 294.739 —— Diethyl (phenylsulfinyl)methylphosphonate 50746-65-1 C11H17O4PS 276.293 —— bisphenylthiomethanephosphonate 73778-53-7 C17H21O3PS2 368.458 —— [Diethoxyphosphoryl(methylsulfanyl)methyl]sulfanylbenzene 73778-52-6 C12H19O3PS2 306.387 对甲苯磺酰甲基磷酸二乙酯 diethyl p-toluenesulfonylmethylphosphonate 60682-95-3 C12H19O5PS 306.32 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— phenylsulfanylmethyl-phosphonic acid monoethyl ester 747407-63-2 C9H13O3PS 232.24 —— diethyl (1-(phenylthio)ethyl)phosphonate 55264-89-6 C12H19O3PS 274.321 —— diethyl (chloro(phenylthio)methyl)phosphonate 59664-75-4 C11H16ClO3PS 294.739 —— Diethyl (phenylsulfinyl)methylphosphonate 50746-65-1 C11H17O4PS 276.293 —— (S)-diethyl (phenylsulfinyl)methylphosphonate 227952-63-8 C11H17O4PS 276.293 —— bisphenylthiomethanephosphonate 73778-53-7 C17H21O3PS2 368.458 苯砜基甲基膦酸二乙酯 diethyl phenylsulfonylmethylphosphonate 56069-39-7 C11H17O5PS 292.293 —— Phenylmercaptomethanphosphonsaeure 89854-56-8 C7H9O3PS 204.186 —— diethyl α,α-difluoro-α-(phenylthio)methylphosphonate 197356-91-5 C11H15F2O3PS 296.275 —— ethyl phenylsufenylmethanephosphonic chloridate 114077-36-0 C9H12ClO2PS 250.686 —— diethyl (α-phenylthio)-3-butenylphosphonate 194999-08-1 C14H21O3PS 300.359 —— (1-Phenylthio-3-butinyl)phosphonsaeure-diethylester 108298-17-5 C14H19O3PS 298.343 —— [Diethoxyphosphoryl(ethoxy)methyl]sulfanylbenzene 59590-51-1 C13H21O4PS 304.347 —— isopropylthio(phenylthio)methanephosphonate 123277-92-9 C14H23O3PS2 334.441 —— bis(diethylphosphoryl)phenylthiomethane 73778-50-4 C15H26O6P2S 396.381 —— phenylthio(n-propylthio)methanephosphonate 123277-91-8 C14H23O3PS2 334.441 —— isobutylthio(phenylthio)methanephosphonate 123277-93-0 C15H25O3PS2 348.467 - 1
- 2
反应信息
-
作为反应物:描述:二乙基(苯基硫代甲基)磷酸酯 在 三氟化硼乙醚 、 1-(过氧化叔丁基)-1,2-苯碘酰-3(H)-酮 作用下, 以 水 、 乙腈 为溶剂, 反应 38.0h, 以82%的产率得到Diethyl (phenylsulfinyl)methylphosphonate参考文献:名称:用高价(叔丁基过氧)碘将硫化物氧化成亚砜。摘要:在乙腈-水中或二氯甲烷中,用结晶的(烷基过氧)碘化物,1-(叔丁基过氧)-1,2-苯并三恶唑-3(1H)-酮2a和2b氧化硫化物,可高产率提供亚砜。在乙腈水中测量一系列环取代的硫代苯甲醚3b(p-MeO),3c(p-Me)和3d(p-Cl)的相对氧化速率表明,电子释放基团(例如p- MeO和p-Me基团增加了氧化速率,相对速率因子与取代基常数的Hammett相关性提供了BF(3)的反应常数rho(+)= -2.23(sigma(+),r = 0.98)催化氧化,对于未催化氧化,rho = -3.32(sigma,r = 0.98)。检查了自由基清除剂加尔维诺尔的作用。对于存在和不存在BF(3)的乙腈水中的氧化,提出了一种机制,该机制涉及通过硫化物对2的碘(III)原子的亲核攻击而中间形成formation物种11。Et(2)O。另一方面,二氯甲烷中亚砜的氧化可能是通过自由基过程进行的,该过DOI:10.1021/jo970081q
-
作为产物:参考文献:名称:Arylthiovinylcyclopropanecarboxylate insecticides摘要:揭示了具有一般公式##STR1##的芳基硫乙烯基环丙烷羧酸酯。还描述和举例了这些化合物及其新颖中间体的杀虫效果和制备方法。公开号:US04200644A1
文献信息
-
Isomerisation of Vinyl Sulfones for the Stereoselective Synthesis of Vinyl Azides作者:Niall Collins、Robert Connon、Goar Sánchez‐Sanz、Paul EvansDOI:10.1002/ejoc.202001065日期:2020.10.22The efficient isomerisation of a range of γ‐azido substituted vinyl sulfones is reported. The corresponding vinyl azides are typically obtained with high levels of Z‐stereochemistry. An exception is when R is an α‐methyl substituent is present when the E‐vinyl azide was preferred.
-
Chemical introduction of the green fluorescence: imaging of cysteine cathepsins by an irreversibly locked GFP fluorophore作者:Maxim Frizler、Ilia V. Yampolsky、Mikhail S. Baranov、Marit Stirnberg、Michael GütschowDOI:10.1039/c3ob41341a日期:——An activity-based probe, containing an irreversibly locked GFP-like fluorophore, was synthesized and evaluated as an inhibitor of human cathepsins and, as exemplified with cathepsin K, it proved to be suitable for ex vivo imaging and quantification of cysteine cathepsins by SDS-PAGE.
-
Peptidyl allyl sulfones: a new class of inhibitors for clan CA cysteine proteases作者:Marion G. Götz、Conor R. Caffrey、Elizabeth Hansell、James H. McKerrow、James C. PowersDOI:10.1016/j.bmc.2004.07.016日期:2004.10series of peptidyl allyl sulfone inhibitors was discovered while trying to synthesize epoxy sulfone inhibitors from vinyl sulfones using basic oxidizing conditions. The various dipeptidyl allyl sulfones were evaluated with calpain I, papain, cathepsins B and L, cruzain and rhodesain and found to be potent inhibitors. In comparison to the previously developed class of vinyl sulfone inhibitors, the novel
-
Stereoselective Synthesis of 2-Deoxy-2-iodo-glycosides from Furanoses. A New Route to 2-Deoxy-glycosides and 2-Deoxy-oligosaccharides of <i>r</i><i>ibo </i>and <i>x</i><i>ylo</i> Configuration作者:Miguel Ángel Rodríguez、Omar Boutureira、Xavier Arnés、M. Isabel Matheu、Yolanda Díaz、Sergio CastillónDOI:10.1021/jo051461b日期:2005.12.1procedure involves three reactions: Wittig−Horner olefination to give alkenyl sulfanyl derivatives, electrophilic iodine-induced cyclization to give phenyl 2-deoxy-2-iodo-1-thio-hexo-glycosides, and glycosylation. Protected furanoses 1, 3, and 6−11, which include examples of the four possible isomeric configurations of furanoses, were reacted with diphenyl phenylsulfanylmethyl phosphine oxide to give the据报道从呋喃糖酶立体选择性合成2-脱氧-2-碘-己-和-庚-吡喃糖基糖苷的一般方法。拟议的方法提供了一种获取2-脱氧寡糖的新途径。该程序涉及三个反应:Wittig-Horner烯化生成链烯基硫烷基衍生物,亲电碘诱导的环化生成苯基2-脱氧-2-碘-1-硫代-己糖苷,以及糖基化。保护呋喃糖1,3,和6 - 11,其包括呋喃糖的四种可能的异构体构型的实施例中,与碳酸二苯苯基硫基甲基氧化膦,得到链烯基硫烷基衍生物进行反应2,4,和12− 16。这些化合物,得到苯基2-脱氧-2-碘-1-硫代糖苷的碘诱导的环化18,20,和22 - 27具有实际上完全区域选择性和立体选择性。6-产品内的环化,其中在C-2中的碘是一个顺式在C-3与烷氧基的关系,进行了几乎全部产生。具有核糖或木糖构型的化合物比具有其他构型的化合物获得更好的产率。化合物18,20,和22 - 27已发现在胆固醇和吡喃葡萄糖苷29a的糖基化
-
Peptidyl allyl sulfones申请人:Powers C. James公开号:US20060241057A1公开(公告)日:2006-10-26The present disclosure provides compositions for inhibiting proteases, methods for synthesizing the compositions, and methods of using the disclosed protease inhibitors. Aspects of the disclosure include peptidyl allyl sulfone compositions that inhibit proteases, for example cysteine proteases, either in vivo or in vitro.
表征谱图
-
氢谱1HNMR
-
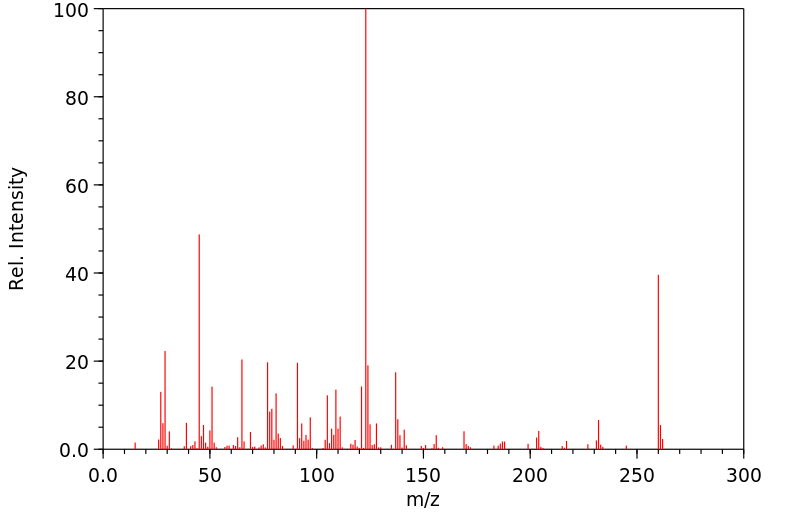
质谱MS
-
碳谱13CNMR
-
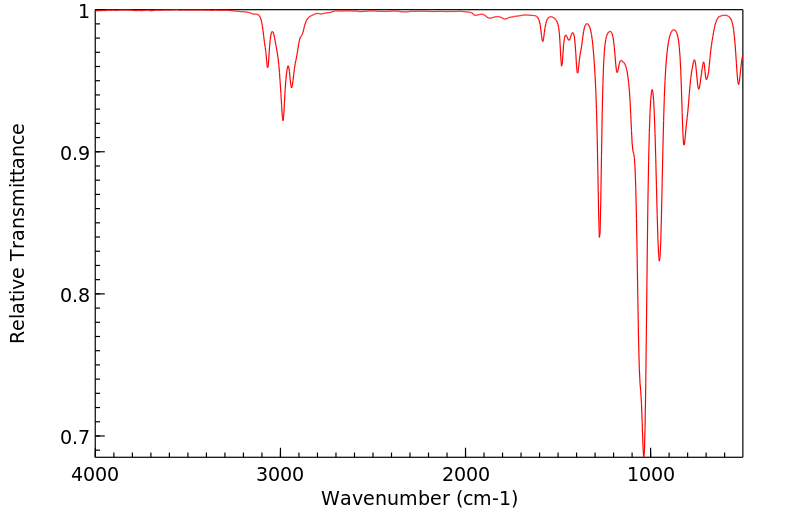
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(Rp)-2-(叔丁硫基)-1-(二苯基膦基)二茂铁
(1E)-1-{4-[(4-氨基苯基)硫烷基]苯基}乙酮肟
颜料红88
颜料紫36
顺式-1,2-二(乙硫基)-1-丙烯
非班太尔-D6
雷西那得中间体
阿西替尼杂质J
阿西替尼杂质C
阿西替尼杂质4
阿西替尼杂质
阿西替尼
阿拉氟韦
阿扎毒素
阿嗪米特
阔草特
银(I)(6-氨基-2-(甲硫基)-5-亚硝基嘧啶-4-基)酰胺水合物
钾三氟[3-(苯基硫基)丙基]硼酸酯(1-)
邻甲苯基(对甲苯基)硫化物
避虫醇
连翘脂苷B
还原红 41
还原紫3
还原桃红R
达索尼兴
辛硫醚
辛-1,7-二炔-1-基(苯基)硫烷
西嗪草酮
萘,2-[(2,3-二甲基苯基)硫代]-
莫他哌那非
茴香硫醚
苯醌B
苯酰胺,N-(氨基亚氨基甲基)-4-[(2-甲基苯基)硫代]-3-(甲磺酰)-,盐酸盐
苯酰胺,N-(氨基亚氨基甲基)-4-[(2-氯苯基)硫代]-3-(甲磺酰)-,盐酸盐
苯酰胺,N-(氨基亚氨基甲基)-4-[(2,6-二氯苯基)硫代]-3-(甲磺酰)-,盐酸盐
苯酰胺,2-[(2-硝基苯基)硫代]-
苯酚,3-氯-4-[(4-硝基苯基)硫代]-
苯酚,3-(乙硫基)-
苯酚,3,5-二[(苯基硫代)甲基]-
苯胺,4-[5-溴-3-[4-(甲硫基)苯基]-2-噻嗯基]-
苯胺,3-氯-4-[(1-甲基-1H-咪唑-2-基)硫代]-
苯胺,2-[(2-吡啶基甲基)硫代]-
苯硫醚-D10
苯硫胍
苯硫基乙酸
苯硫代磺酸S-(三氯乙烯基)酯
苯甲醇,2,3,4,5,6-五氟-a-[(苯基硫代)甲基]-,(R)-
苯甲酸,3-[[2-[(二甲氨基)甲基]苯基]硫代]-,盐酸
苯甲胺,5-氟-2-((3-甲氧苯基)硫代)-N,N-二甲基-,盐酸
苯甲二硫酸,4-溴苯基酯