亞甲去甲 | 694-91-7
中文名称
亞甲去甲
中文别名
亞甲去甲[0XA29C];5-亚甲基-2-降冰片烯
英文名称
5-methylenebicyclo[2.2.1]hept-2-ene
英文别名
5-methylene-2-norbornene;5-methylenenorbornene;5-Methylen-2-norbornen;5-methylenebicyclo<2.2.1>hept-2-ene;5-Methylen-bicyclo<2.2.1>hept-2-en;2-methylenebicyclo<2.2.1>hept-5-ene;2-Methyliden-norborn-5-en;5-Methylennorbornen;5-methylidenebicyclo[2.2.1]hept-2-ene
CAS
694-91-7
化学式
C8H10
mdl
——
分子量
106.167
InChiKey
WTQBISBWKRKLIJ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:116 °C
-
密度:0.8909 g/cm3
-
保留指数:798;800;800;803
计算性质
-
辛醇/水分配系数(LogP):2
-
重原子数:8
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
危险等级:3.1
-
危险品运输编号:UN 1993
-
包装等级:II
-
危险类别:3.1
SDS
上下游信息
反应信息
-
作为反应物:描述:亞甲去甲 660.0~690.0 ℃ 、266.64 Pa 条件下, 生成 3-Methylen-cycloheptadien-(1,4)参考文献:名称:Thermal Rearrangement of 5-Methylenebicyclo [2.2.1]hept-2-ene1摘要:DOI:10.1021/ja01496a045
-
作为产物:参考文献:名称:Versatile allene and carbon dioxide equivalents for the Diels-Alder reaction摘要:DOI:10.1021/jo00445a024
文献信息
-
Synthetic Photochemistry. LX. One-pot Formation of Spirocyclic 3-Acetyl-2-hydroxy-2-cyclopentenone Derivatives from Methylenecycloalkanes and Methyl 2,4-Dioxopentanoate作者:Toshihide Hatsui、Shin-ya Ikeda、Hitoshi TakeshitaDOI:10.1246/bcsj.66.870日期:1993.3of proto-photocycloadducts of methyl 2,4-dioxopentanoate to methylenecycloalkanes and -alkenes spontaneously caused retro-benzilic acid rearrangement in high yields. The results are utterly different from those of sterically-crowded acyclic alkenes, with which the rearrangement occurred by thermolysis as a minor process.
-
Scope, limitations and mechanistic aspects in the selective homogeneous palladium-catalyzed reduction of alkenes under transfer hydrogen conditions作者:Jean Michel BrunelDOI:10.1016/j.tet.2007.01.053日期:2007.4A new and efficient mild Pd/P(t-Bu)3 catalyst for selective reduction of various alkenes under transfer hydrogen conditions has been developed leading to the corresponding saturated derivatives in chemical yields varying from 65 to 98%. Mechanistic rationale of this reaction has been also demonstrated.
-
Hexahalocyclopentadienes—III作者:R.G. Pews、C.W. Roberts、C.R. HandDOI:10.1016/s0040-4020(01)93021-2日期:1970.1hexabromocyclopentadiene (1) with sodium methoxide in methanol-glyme provides 1,2,3,4-tetrabromo-5,5-dimethoxycyclopentadiene (6). The reactivity of this new diene in the Diels-Alder reaction has been determined with a number of dienophiles. The greater reactivity of ketal 6 towards maleic anhydride compared to cyclopentadiene suggests that 6 behaves as an electron-rich diene in the Diels-Alder reaction.
-
Rotational correlation times and radii of dithiazol-2-yl and dithiazolidin-2-yl free radicals作者:Shirley A. Fairhurst、Roger S. Pilkington、Leslie H. SutcliffeDOI:10.1039/f19837900439日期:——2-dithiazolidin-2-yl radicals have been shown to be useful model compounds for detailed e.s.r. studies of rotational reorientation in liquid and frozen media. Consequently they are also potentially useful spin probes and spin labels. The e.s.r. powder spectra have been analysed and the resulting g and A tensors were used to calculate rotational diffusion correslation times, rotational energetics of activation
-
Gold<i>versus</i>Silver-Catalyzed Intermolecular Hydroaminations of Alkenes and Dienes作者:Xavier Giner、Carmen Nájera、Gábor Kovács、Agustí Lledós、Gregori UjaqueDOI:10.1002/adsc.201100478日期:2011.12Comparative studies about the hydroamination of unactivated alkenes and dienes catalyzed by either cationic gold(I) triphenyl phosphite complexes or silver salts were performed using sulfonamides, anilines and carbamates as nucleophiles. Gold-catalyzed reactions generally, need lower loadings than those carried out with silver salts. Simple alkenes react only with sulfonamides and weak aromatic amines使用磺酰胺,苯胺和氨基甲酸酯作为亲核试剂,对阳离子金(I)亚磷酸三苯酯配合物或银盐催化的未活化烯烃和二烯的加氢胺化进行了比较研究。与银盐进行的反应相比,金催化的反应通常需要较低的负载量。简单烯烃仅与磺酰胺和弱芳族胺(例如对位)反应-硝基苯胺,而对于共轭二烯,也可以使用氨基甲酸酯。碳-碳双键异构化仅在使用金的情况下才能观察到,与使用三氟甲磺酸相似,在相同情况下可提供区域异构体产物的混合物。银催化的加氢胺化反应除苯乙烯外均不能用于末端烯烃。共轭二烯可以在85°C的甲苯中或室温下的二氯甲烷中进行氨化。非共轭的1,4-和1,5-二烯经历两次加氢胺化反应,生成饱和的N-甲苯磺酸化杂环胺。已对银(I)催化的加氢胺化过程的催化循环进行了计算分析,类似于金(I)催化的过程。 ,尽管有一些显着差异。
表征谱图
-
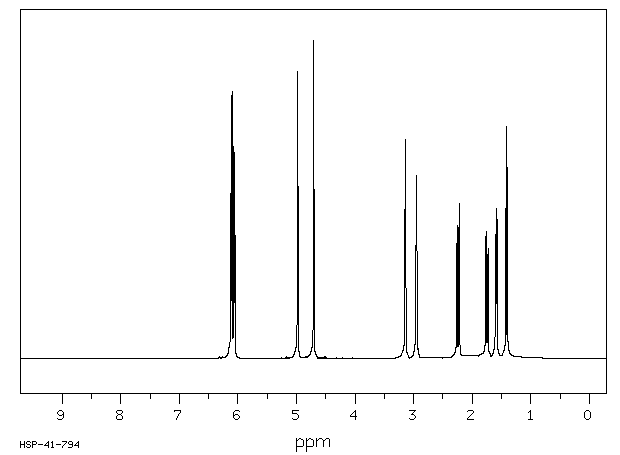
氢谱1HNMR
-
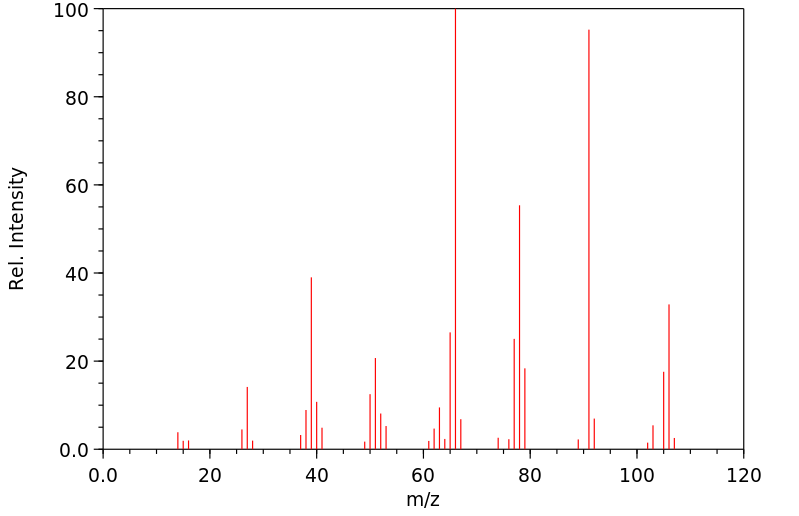
质谱MS
-
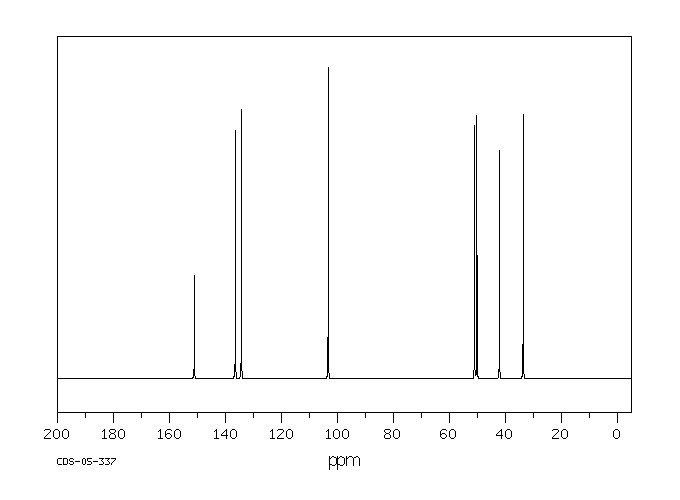
碳谱13CNMR
-
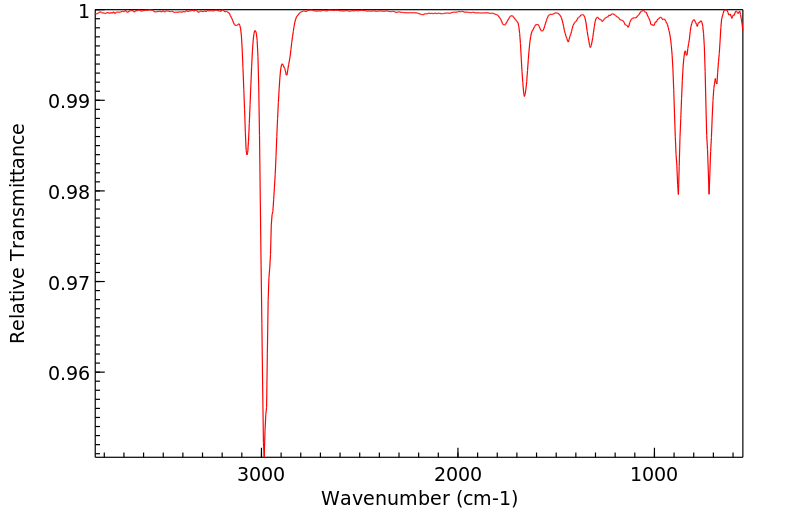
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-