十二烷酸二乙酯 | 10471-28-0
中文名称
十二烷酸二乙酯
中文别名
十二烷二酸二乙酯
英文名称
diethyl dodecanedioate
英文别名
——
CAS
10471-28-0
化学式
C16H30O4
mdl
——
分子量
286.412
InChiKey
AFYNPRWLOKYLDU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:15 °C(lit.)
-
沸点:192-193 °C14 mm Hg(lit.)
-
密度:0.951 g/mL at 25 °C(lit.)
-
闪点:>230 °F
-
溶解度:易溶于可溶于氯仿、甲醇
-
稳定性/保质期:
遵照规格使用和储存,则不会分解。
计算性质
-
辛醇/水分配系数(LogP):4.6
-
重原子数:20
-
可旋转键数:15
-
环数:0.0
-
sp3杂化的碳原子比例:0.88
-
拓扑面积:52.6
-
氢给体数:0
-
氢受体数:4
安全信息
-
WGK Germany:3
-
海关编码:2917190090
-
储存条件:密封于阴凉干燥处。
SDS
十二烷二酸二乙酯 修改号码:2
模块 1. 化学品
产品名称: Diethyl Dodecanedioate
修改号码: 2
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 十二烷二酸二乙酯
百分比: >95.0%(GC)
CAS编码: 10471-28-0
俗名: Dodecanedioic Acid Diethyl Ester
分子式: C16H30O4
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
十二烷二酸二乙酯 修改号码:2
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。处理后彻底清洗双手
和脸。
注意事项: 如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-浅黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度: 无资料
模块 10. 稳定性和反应性
稳定性: 一般情况下稳定。
十二烷二酸二乙酯 修改号码:2
模块 10. 稳定性和反应性
反应性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布): 针对危险化学品的安全使用、生产、储存、运输、装
卸等方面均作了相应的规定。
十二烷二酸二乙酯 修改号码:2
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: Diethyl Dodecanedioate
修改号码: 2
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 十二烷二酸二乙酯
百分比: >95.0%(GC)
CAS编码: 10471-28-0
俗名: Dodecanedioic Acid Diethyl Ester
分子式: C16H30O4
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
十二烷二酸二乙酯 修改号码:2
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。处理后彻底清洗双手
和脸。
注意事项: 如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-浅黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度: 无资料
模块 10. 稳定性和反应性
稳定性: 一般情况下稳定。
十二烷二酸二乙酯 修改号码:2
模块 10. 稳定性和反应性
反应性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布): 针对危险化学品的安全使用、生产、储存、运输、装
卸等方面均作了相应的规定。
十二烷二酸二乙酯 修改号码:2
模块16 - 其他信息
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 十二烷二酸 1,12-Dodecanedioic acid 693-23-2 C12H22O4 230.304 —— 2,2'-Dibrom-dodecandisaeure-diethylester 22400-00-6 C16H28Br2O4 444.204 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 十二烷二酸一乙酯 dodecanedioic acid monoethyl ester 66003-63-2 C14H26O4 258.358 —— 1-carboxytridecyl 14-hydroxytridecanoate 78651-84-0 C28H54O5 470.734 十二烷二酸单甲酯 12-methoxy-12-oxododecanoic acid 3903-40-0 C13H24O4 244.331 十四烷二酸 1,14-tetradecanedioic acid 821-38-5 C14H26O4 258.358
反应信息
-
作为反应物:参考文献:名称:Synthesis and pharmacological properties of bisquaternary salts of α,ω-Di[N-methyl-N-(1-adamantyl)amino] alkanes摘要:DOI:10.1007/bf00771468
-
作为产物:描述:6-(4-甲基苯基)磺酰氧基己酸乙酯 在 锰 、 三甲基氯硅烷 、 vitamin B12 、 1,10-邻菲啰啉二溴化镍 作用下, 以 二甲基亚砜 为溶剂, 反应 24.0h, 以70%的产率得到十二烷酸二乙酯参考文献:名称:Ni/Co 催化的甲苯磺酸烷基酯均偶联反应摘要:已经通过使用镍和亲核钴催化剂的组合开发了甲苯磺酸烷基酯的直接还原均偶联。单电子转移型氧化加成是完善的镍催化烷基卤化物偶联的关键过程。然而,由于甲苯磺酸酯的高位 σ*(C-O) 轨道,该方法不能应用于普遍存在的甲苯磺酸酯烷基酯的均偶联。本文描述了一种 Ni/Co 催化的协议,用于在温和条件下构建烷基二聚体时活化甲苯磺酸烷基酯。DOI:10.3390/molecules24081458
文献信息
-
Amphiphilic Anionic Analogues of Galactosylceramide: Synthesis, Anti-HIV-1 Activity, and gp120 Binding作者:Barbara Faroux-Corlay、Jacques Greiner、Raphaël Terreux、Daniel Cabrol-Bass、Anne-Marie Aubertin、Pierre Vierling、Jacques FantiniDOI:10.1021/jm0011124日期:2001.6.1structure contains also an amino group or several hydroxyls or anionic groups, such as carboxylate, sulfate, sulfonate, and phosphate. Among the 12 new galactosylated compounds reported, a specific anti-HIV activity, although moderate (IC(50) from 10 to 50 microM), was detected only for three of them, i.e., I-GalSer[CO2Na][C14], II-GalSer[C14][C7SO3Na], and II-GalSer[C2SO4Na][C14], which contain an anionic
-
One-pot synthesis of cyclophane-type macrocycles using manganese(iii)-mediated oxidative radical cyclization作者:Yosuke Ito、Yuichi Tomiyasu、Takahiro Kawanabe、Keisuke Uemura、Yuu Ushimizu、Hiroshi NishinoDOI:10.1039/c0ob00683a日期:——Cyclophane-type macrocyclic compounds from 21 to 56 members having two fused dihydrofuran rings were synthesized by the manganese(III)-mediated oxidation of terminal dienes with bis(3-oxobutanoate)s containing aromatics. The reaction detail, characterization and reaction pathways are described.
-
Cyclic Acetylenes. XI. The Syntheses of<i>p</i>,<i>p</i>′-Bridged Cyclic Tolans by the Fritsch-Buttenberg-Wiechell Rearrangement作者:Takashi Ando、Masazumi NakagawaDOI:10.1246/bcsj.44.172日期:1971.1In order to ascertain the effect of ring strain on the electronic spectra of cyclic acetylenes, a series of p,p′-bridged cyclic tolans (Vn, n=11, 12, 13, 14, and 18) has been synthesized. It was found that the increasing ring strain exerted an appreciable hypochromic effect and also caused a slight bathochromic shift of the absorption band. The bathochromic shift observed in the p,p′-bridged cyclic tolans (Vn) forms a contrast with the hypsochromic shift observed in the strained o,o′-bridged cyclic diphenyldiacetylene (VI, n=3).为了确定环张力对环状炔烃电子光谱的影响,合成了一系列p,p′-桥接的环状托烯(Vn,n=11、12、13、14和18)。结果发现,增加的环张力产生了显著的淡色效应,并导致了吸收带的轻微红移。在p,p′-桥接的环状托烯(Vn)中观察到的红移与在受张力的o,o′-桥接环状二苯二炔(VI,n=3)中观察到的蓝移形成了对比。
-
A solid-state NMR study of the 2,15-dimethylhexadecane/urea inclusion compound作者:Thomas Handel、Falk Lissner、Thomas Schleid、Klaus MüllerDOI:10.1016/j.molstruc.2006.10.029日期:2007.6the channel long axis, which is a major source for spin-lattice relaxation. Moreover, it is found that the orientation of the methyl groups is perpendicular to the long molecular axis (i.e. gauche conformation at both chain ends) which gives rise to a better molecular packing and stronger van-der-Waals interactions. Activation energies of 24.3 (±1.0) kJ/mol are derived for the overall molecular rotation摘要 首次对具有双支链烷烃客体分子的尿素包合物进行了固态 2 H- 和 13 C NMR 研究。在两个亚甲基(C3 和 C14 位置)或两个末端异丙基(C1、C1'、C2、C15、C16 和 C16' 位置)上氘化的 2,15-二甲基十六烷的两个样品上进行了变温 2 H NMR 测量)。13 C NMR 数据表明内链段存在于反式构象中。对实验 2 H NMR 数据的综合分析表明,客体分子围绕通道长轴进行受限制的整体旋转(以简并 2 位点跳跃过程建模),这是自旋晶格弛豫的主要来源。而且,发现甲基的方向垂直于分子长轴(即两个链端的 gauche 构象),这会产生更好的分子堆积和更强的范德华相互作用。整个分子旋转的活化能为 24.3 (±1.0) kJ/mol,而甲基旋转值为 14.4 (±0.6) kJ/mol(210 和 250 K 之间)和 12.0 (±1.7) kJ/mol (在 160
-
A new route to α,ω-diamines from hydrogenation of dicarboxylic acids and their derivatives in the presence of amines作者:Yiping Shi、Paul C. J. Kamer、David J. Cole-HamiltonDOI:10.1039/c7gc02838e日期:——A new and selective route for the synthesis of polymer precursors, primary diamines or N-substituted diamines, from dicarboxylic acids, diesters, diamides and diols using a Ru/ triphos catalyst is reported. Excellent conversions and yields are obtained under optimised reaction conditions. The reactions worked very well using 1,4-dioxane as solvent, but the greener solvent, 2-methyl tetrahydrofuran
表征谱图
-
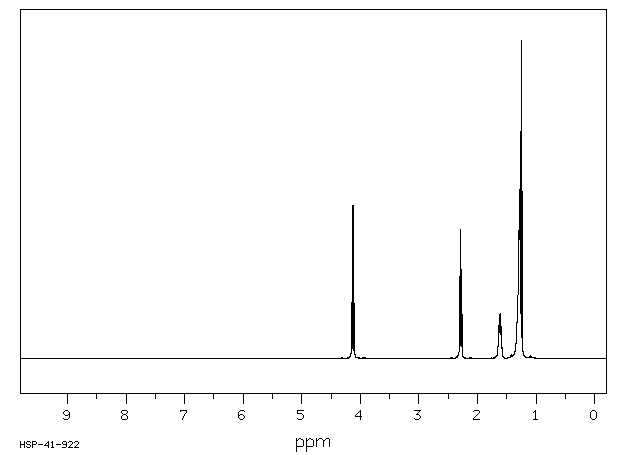
氢谱1HNMR
-
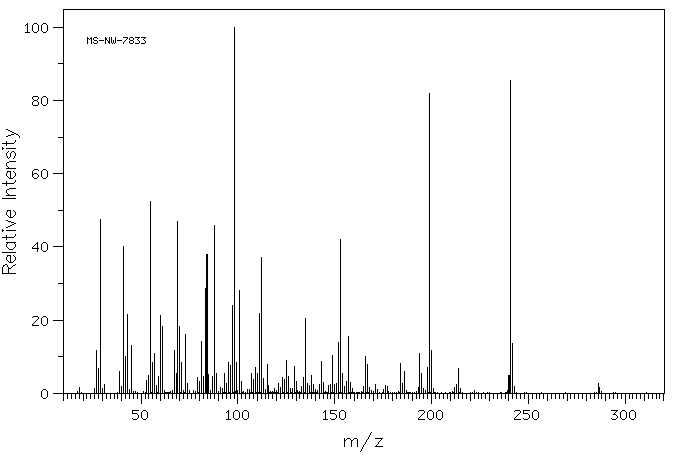
质谱MS
-
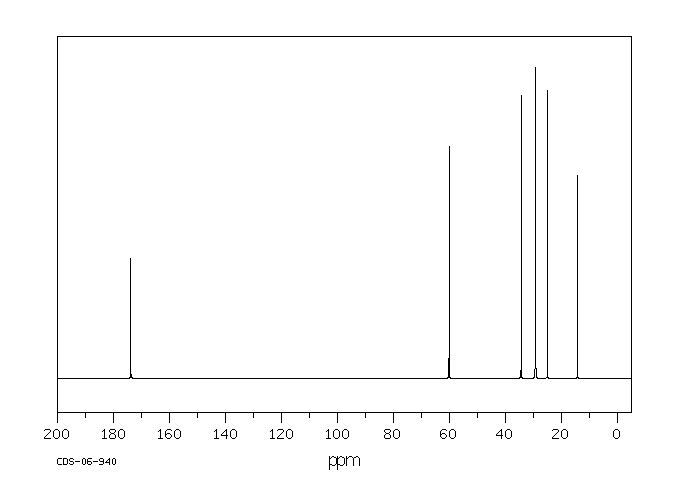
碳谱13CNMR
-
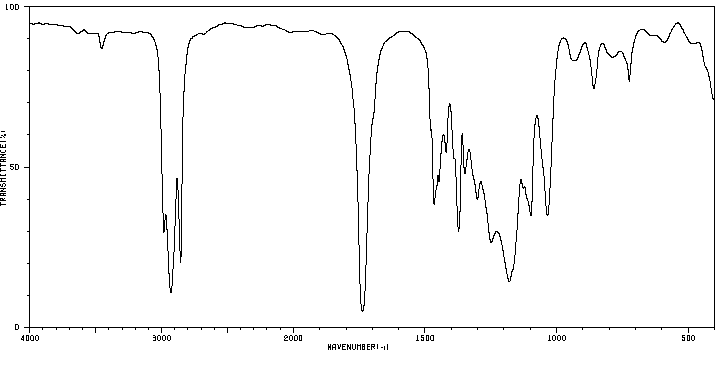
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯