十五烷-5-酮 | 92862-23-2
中文名称
十五烷-5-酮
中文别名
——
英文名称
pentadecan-5-one
英文别名
Pentadecan-5-on;5-Pentadecanone
CAS
92862-23-2
化学式
C15H30O
mdl
——
分子量
226.403
InChiKey
NADMBULPSVEFAV-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:35.25°C (estimate)
-
沸点:293.03°C (estimate)
-
密度:0.8184 (estimate)
计算性质
-
辛醇/水分配系数(LogP):6
-
重原子数:16
-
可旋转键数:12
-
环数:0.0
-
sp3杂化的碳原子比例:0.93
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
SDS
反应信息
-
作为反应物:参考文献:名称:通过光诱导的HAT引发分子间烯烃加氢酰化的一般方法:长链脂肪族酮和功能化脂肪酸的高效合成摘要:本文介绍了一种通过使用光诱导氢原子转移(HAT)引发对未活化底物进行分子间自由基加氢酰化的操作简单,对环境无害且有效的方法。使用可商购和廉价的光引发剂(Ph 2 CO和NHPI)使该方法具有吸引力。烯烃加氢酰化方案适用于多种带有许多官能团和许多复杂结构单元的底物。该反应证明是可扩展的(最大5 g)。可以使用该方案合成不同的官能化脂肪酸,石化产品和天然存在的烷烃。自由基链机制与该过程有关。DOI:10.1002/chem.202004946
-
作为产物:参考文献:名称:Electrochemical oxidation of acylsilanes and their tosylhydrazones摘要:Oxidation potentials of acylsilanes were found to be much less positive than those of ketones and aldehydes. The effect of silicon is attributed to the rise of the HOMO level by the interaction between the C-Si sigma-orbital and the nonbonding p orbital of the carbonyl oxygen which in turn favors the electron transfer. Preparative electrochemical oxidation of acylsilanes proceeded smoothly, giving rise to facile cleavage of the C-Si bond and the introduction of nucleophiles such as alcohols, water, and carbamates onto the carbonyl carbon. Electrochemical properties of tosylhydrazones of acylsilanes were also investigated. A decrease in oxidation potential of tosylhydrazones caused by silyl substitution was found to be smaller than that for carbonyl compounds. Preparative electrochemical oxidation of tosylhydrazones of acylsilanes gave the corresponding nitriles with consumption of a catalytic amount of electricity.DOI:10.1021/jo00044a023
文献信息
-
Ruthenium-catalyzed Isomerization of Alkenol into Alkanone in Water under Irradiation of Microwaves作者:Kenichi Ishibashi、Masaaki Takahashi、Yutaka Yokota、Koichiro Oshima、Seijiro MatsubaraDOI:10.1246/cl.2005.664日期:2005.5Ruthenium catalyzed isomerization of alkenol into alkanone through a migration of C–C double bond was performed in water under irradiation of microwaves. When the reaction was performed in deuterium oxide instead of water, the trail of the migration was shown by H–D exchange reaction.
-
Mild and direct conversion of esters to morpholine amides using diisobutyl(morpholino)aluminum: application to efficient one-pot synthesis of ketones and aldehydes from esters作者:Ah Ram Jeon、Min Eai Kim、Jae Kyo Park、Won Kyu Shin、Duk Keun AnDOI:10.1016/j.tet.2014.03.045日期:2014.7Morpholine amide intermediates, which are easily prepared by aminolysis of various esters with diisobutyl(morpholino)aluminum, react with organolithium and reducing agents (DIBALH or LDBMA) without isolation of the aminolysis intermediates to give ketones in 83–95% yields and aldehydes quantitatively.
-
Hydrogen transfer type oxidation of alcohols by rhodium and ruthenium catalyst under microwave irradiation作者:Masaaki Takahashi、Koichiro Oshima、Seijiro MatsubaraDOI:10.1016/j.tetlet.2003.10.032日期:2003.12Secondary alcohols were converted into the corresponding ketones by methyl acrylate and rhodium catalyst efficiently under microwave irradiation. Treatment of primary alcohols with the same condition resulted in the recovery of the starting materials. Primary alcohols were converted into aldehydes by hydrogen transfer reaction using methyl vinyl ketone and ruthenium catalyst under microwave irradiation
-
An effective one-pot conversion of acid chlorides to aldehydes and ketones作者:Jae Kyo Park、Won Kyu Shin、Duk Keun AnDOI:10.1016/j.tetlet.2013.04.041日期:2013.6Aldehydes and ketones were synthesized from their respective acid chlorides via a one-pot protocol. Morpholine amide intermediates that were readily prepared by the aminolysis of various acid chlorides with diisobutyl(morpholino)aluminum smoothly reacted with the reducing agent LDBMA and the organolithium reagents under mild reaction conditions (0 °C), giving almost excellent product yields of up to
-
Chemoselective Conversion of Conjugated Nitroalkenes into Ketones by Sodium Borohydride-Hydrogen Peroxide: A New Synthesis of 4-Oxoalkanoic Acids, Dihydrojasmone and (±)-<i>exo</i>-Brevicomin作者:Roberto Ballini、Giovanna BosicaDOI:10.1055/s-1994-25557日期:——A new, simple, cheap, and practical procedure for the direct transformation of α,β-unsaturated nitroalkenes into ketones has been realized by the NaBH4/H2O2 system. By this method, other functional groups such as C-C double bonds, ketals or aromatic nitro groups were preserved. Application of this methodology to the preparation of 4-oxoalkanoic acids, dihydrojasmone, and (±)-exo-brevicomin is also reported.
表征谱图
-
氢谱1HNMR
-
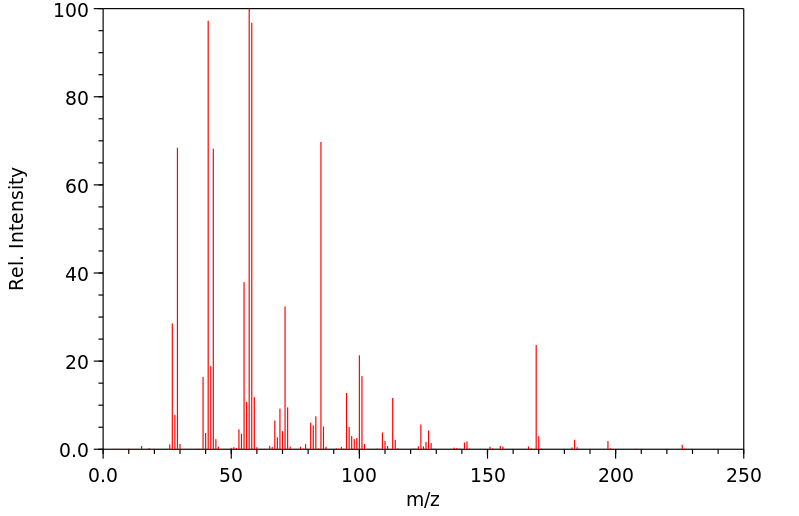
质谱MS
-
碳谱13CNMR
-
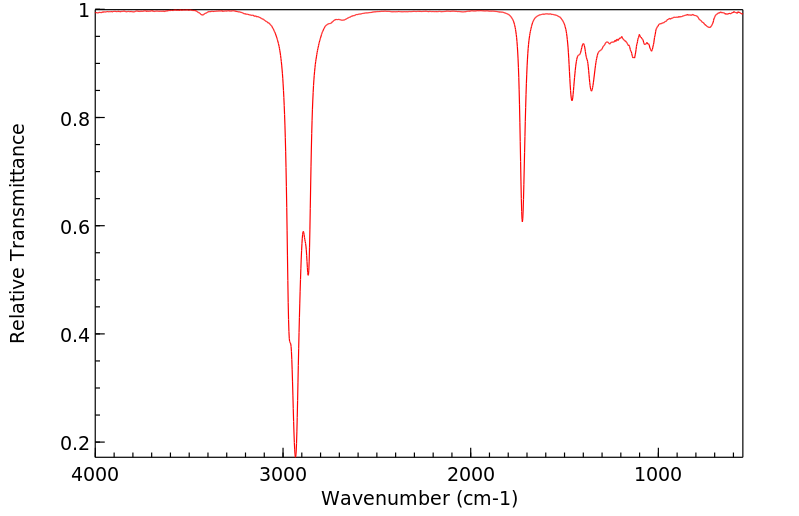
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷