反式 环丙烷-1,2-二羧酸 | 58616-95-8
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:175°C
-
沸点:200.75°C (rough estimate)
-
密度:1.4600
计算性质
-
辛醇/水分配系数(LogP):-0.6
-
重原子数:9
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.6
-
拓扑面积:74.6
-
氢给体数:2
-
氢受体数:4
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1,2-环丙烷二羧酸 cyclopropane-1,2-dicarboxylic acid 1489-58-3 C5H6O4 130.1 —— (±)-trans-2-(hydroxymethyl)cyclopropanecarboxylic acid 35501-81-6 C5H8O3 116.117 —— (1R,2R)-2-(methoxycarbonyl)cyclopropane-1-carboxylic acid 88335-97-1 C6H8O4 144.127 反-1,2-环丙烷二羧酸二甲酯 dimethyl trans-1,2-cyclopropanedicarboxylate 826-35-7 C7H10O4 158.154 —— (+/-)-trans-2-ethoxymethyl-cyclopropanecarboxylic acid —— C7H12O3 144.17 —— Aethylmethyl-1,2-cyclopropandicarboxylat 878-14-8 C8H12O4 172.181 反-1,2-环丙烷二羧酸二乙酯 diethyl trans-cyclopropane-1,2-dicarboxylate 3999-55-1 C9H14O4 186.208 —— 2-acetyl-cyclopropanecarboxylic acid 858423-15-1 C6H8O3 128.128 —— (1R,2R)-ethyl 2-(hydroxymethyl)cyclopropanecarboxylate 1026787-30-3 C7H12O3 144.17 —— (+/-)-(1R,2S)-2-(1-methylethoxycarbonyl)-1-cyclopropanecarboxylic acid —— C8H12O4 172.181 —— cis-1,2-cyclopropanedicarboxylic acid anhydride 5617-74-3 C5H4O3 112.085 —— (-)-(R,R)-2-chloromethylenecyclopropane-1-carboxylic acid 1101900-05-3 C5H7ClO2 134.562 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (1R,2R)-2-(methoxycarbonyl)cyclopropane-1-carboxylic acid 88335-97-1 C6H8O4 144.127 顺-1,2-环丙烷二羧酸二甲酯 dimethyl cis-cyclopropane-1,2-dicarboxylate 826-34-6 C7H10O4 158.154 反-1,2-环丙烷二羧酸二甲酯 dimethyl trans-1,2-cyclopropanedicarboxylate 826-35-7 C7H10O4 158.154 (1R,2R)-2-(乙氧羰基)环丙烷羧酸 trans-cyclopropane-1,2-dicarboxylic acid monoethyl ester 613261-14-6 C7H10O4 158.154 反-1,2-环丙烷二羧酸二乙酯 diethyl trans-cyclopropane-1,2-dicarboxylate 3999-55-1 C9H14O4 186.208 —— cis-1,2-cyclopropanedicarboxylic acid anhydride 5617-74-3 C5H4O3 112.085 3-氧杂二环[3.1.0]己烷-2,4-二酮 3-oxabicyclo[3.1.0]hexane-2,4-dione 5617-74-3 C5H4O3 112.085 —— (1R,2R)-2-(tert-butoxycarbonyl)cyclopropane-1-carboxylic acid 1909288-13-6 C9H14O4 186.208
反应信息
-
作为反应物:参考文献:名称:2.1.1.5 The spatial distribution of earthquake foci摘要:该文档是《Landolt-Börnstein - V组地球物理学》第2卷《固体地球、月球和行星的地球物理学》子卷A的一部分。DOI:10.1007/10201917_18
-
作为产物:参考文献:名称:D'jakonow; Gusewa, Sb. Statei Obshch. Khim., 1953, p. 425,430摘要:DOI:
文献信息
-
[EN] ALKYLBORONIC ACIDS AS ARGINASE INHIBITORS<br/>[FR] ACIDES ALKYLBORONIQUES EN TANT QU'INHIBITEURS D'ARGINASE申请人:GUANGDONG NEWOPP BIOPHARMACEUTICALS CO LTD公开号:WO2020160707A1公开(公告)日:2020-08-13Provided are alkylboronic acids as arginase inhibitors represented by formula (I), or a pharmaceutically acceptable salt, stereoisomer, tautomer, or prodrug thereof and a pharmaceutical composition comprising said compounds.
-
[EN] 2-PYRIDYL CARBOXAMIDE-CONTAINING SPLEEN TYROSINE KINASE (SYK) INHIBITORS<br/>[FR] INHIBITEURS DE TYROSINE KINASE DE LA RATE (SYK) CONTENANT UN 2-PYRIDYL CARBOXAMIDE申请人:MERCK SHARP & DOHME公开号:WO2013052394A1公开(公告)日:2013-04-11The invention provides certain 2-pyridyl carboxamide-containing compounds of the Formula (I) or pharmaceutically acceptable salts thereof, wherein A and B are as defined herein. The invention also provides pharmaceutical compositions comprising such compounds, and methods of using the compounds for treating diseases or conditions mediated by Spleen Tyrosine Kinase (Syk) kinase.
-
Synthesis and Biological Activity of a Novel Class of Small Molecular Weight Peptidomimetic Competitive Inhibitors of Protein Tyrosine Phosphatase 1B作者:Scott D. Larsen、Tjeerd Barf、Charlotta Liljebris、Paul D. May、Derek Ogg、Theresa J. O'Sullivan、Barbara J. Palazuk、Heinrich J. Schostarez、F. Craig Stevens、John E. BleasdaleDOI:10.1021/jm010393s日期:2002.1.1sulfate with other potential phosphate mimics. The most potent analogue arising from this effort was triacid 71, which inhibits PTP1B competitively with a K(i) = 0.22 microM without inhibiting SHP-2 or LAR at concentrations up to 100 microM. Overall, the inhibitors generated in this work showed little or no enhancement of insulin signaling in cellular assays. However, potential prodrug triester 70 did induce蛋白质酪氨酸磷酸酶1B(PTP1B)部分地通过使胰岛素受体(IR)的β亚基调节域内的关键酪氨酸残基去磷酸化,从而负调节胰岛素信号传导,从而减弱受体酪氨酸激酶的活性。因此,抑制PTP1B有望改善胰岛素抵抗,并且最近已成为旨在鉴定用于治疗II型糖尿病的新药物的发现工作的重点。我们以前曾报道三肽Ac-Asp-Tyr(SO(3)H)-Nle-NH(2)是PTP1B的令人惊讶的有效抑制剂(K(i)= 5 microM)。为了改善该引线的稳定性和效力以及减弱其肽特性,进行了模拟程序。该程序初始阶段的具体内容包括用非氨基酸成分替换N和C末端,修饰酪氨酸亚基以及用其他潜在的磷酸盐模拟物替换硫酸酪氨酸。从这种努力中产生的最有效的类似物是三酸71,它以K(i)= 0.22 microM竞争性抑制PTP1B,而在浓度高达100 microM的情况下却不抑制SHP-2或LAR。总体而言,这项工作中产生的抑制剂在细
-
The Synthesis of Some Head to Head Linked DNA Minor Groove Binders作者:Abedawn I. Khalaf、Andrew R. Pitt、Martin Scobie、Colin J. Suckling、John Urwin、Roger D. Waigh、Robert V. Fishleigh、Steven C. Young、William A. WylieDOI:10.1016/s0040-4020(00)00432-4日期:2000.7A series of head to head linked dimers of heterocyclic amino acids has been prepared for investigation of affinity and selectivity in binding to the minor groove of DNA. The selection of targets for synthesis was led by computer based design. Several novel dicarboxylic acid linkers including indoles, phenanthrenes, a fluorenone, and a bisbenzothiophene have been included. Analysis of binding to DNA
-
Photodecarbonylation of chiral cyclobutanones作者:Jailall Ramnauth、Edward Lee-RuffDOI:10.1139/v97-060日期:1997.5.1
Triplet photosensitized irradiation of 2(S),3(R)-bis[(benzoyloxy)methyl]cyclobutanone gave optically pure (−)E-1(S),2(S)-bis(benzoyloxymethyl)cyclopropane as a major product in the nonpolar fraction along with its stereoisomer and cycloelimination products. The absolute stereochemistry of the chiral cyclopropane was established by independent synthesis and X-ray crystal structure determination of a synthetic precursor. The distribution of decarbonylation and cycloelimination products was inversely dependent on the concentration of the substrate. Irradiation of the same ketone in tetrahydrofuran or benzene gave mostly cycloelimination products. Addition of Michler's ketone increased the ratio of photodecarbonylation, suggesting a triplet state pathway for this process. This was corroborated by the addition of dicyanoethylene, which showed significant quenching of photodecarbonylation. Irradiation of 2(S)-[(benzoyloxy)methyl]cyclobutane in acetone gave the corresponding cyclopropane as the principal product. Keywords: photodecarbonylation, chiral cyclopropanes, cyclobutanones, triplet sensitization.
三重光敏辐照2(S),3(R)-双[(苯甲酰氧基)甲基]环丁酮,在非极性分馏物中主要生成光学纯的(−)E-1(S),2(S)-双(苯甲酰氧基甲基)环丙烷,同时还有其立体异构体和环消除产物。手性环丙烷的绝对立体化学通过独立合成和合成前体的X射线晶体结构测定来确定。脱羰基化和环消除产物的分布与底物浓度呈反比关系。在四氢呋喃或苯中辐照相同的酮主要生成环消除产物。添加米克勒酮增加了光解脱羰作用的比例,表明了这一过程的三重态途径。通过添加二氰乙烯来证实这一点,显示了光解脱羰作用的显著猝灭。在丙酮中辐照2(S)-[(苯甲酰氧基)甲基]环丁烷产生相应的环丙烷作为主要产物。关键词:光解脱羰作用,手性环丙烷,环丁酮,三重敏化。
表征谱图
-
氢谱1HNMR
-
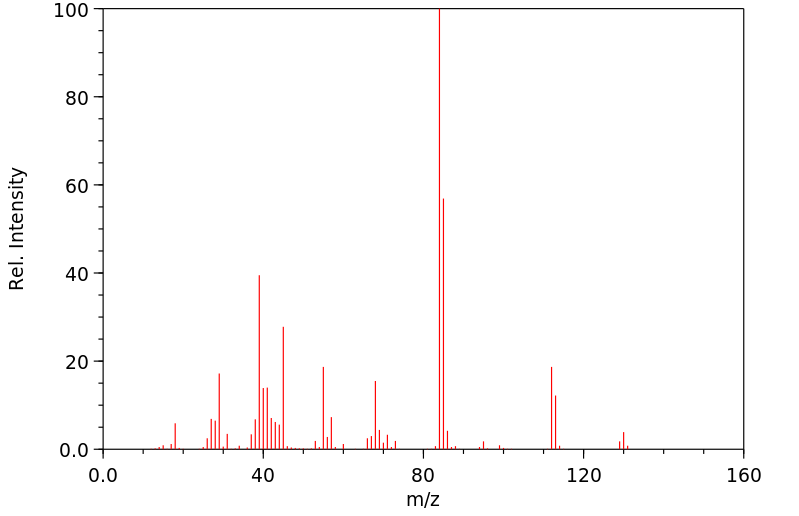
质谱MS
-
碳谱13CNMR
-
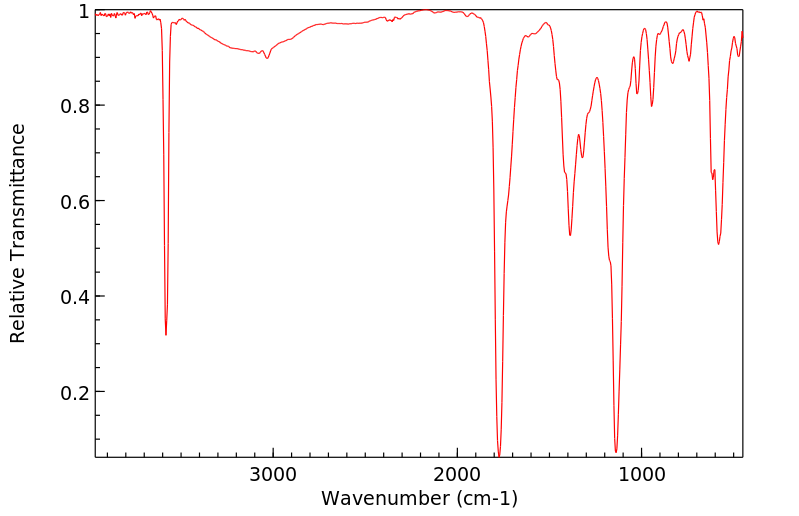
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息