反式-2-己烯醛二乙缩醛 | 67746-30-9
中文名称
反式-2-己烯醛二乙缩醛
中文别名
反-2-己烯-1-醇二乙缩醛;1,1-二乙氧基-反-2-己烯;反-2-己烯醛二乙缩醛;反-2-己烯醛二乙基乙缩醛
英文名称
(E)-1,1-Diethoxy-hex-2-ene
英文别名
trans-2-hexen-1-al diethyl acetal;trans-2-hexenal diethyl acetal;1,1-Diethoxyhex-2-ene;2-Hexene, 1,1-diethoxy-, (2E)-;(E)-1,1-diethoxyhex-2-ene
CAS
67746-30-9
化学式
C10H20O2
mdl
——
分子量
172.268
InChiKey
WMQKYHTZGYIHHD-CMDGGOBGSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:95-98 °C35 mm Hg(lit.)
-
密度:0.848 g/mL at 25 °C(lit.)
-
闪点:145 °F
-
LogP:3.30
-
物理描述:Liquid
-
溶解度:Soluble in most fixed oils; Insoluble in water
-
折光率:1.418-1.426
-
保留指数:1285;1287;1285;1287
计算性质
-
辛醇/水分配系数(LogP):2.6
-
重原子数:12
-
可旋转键数:7
-
环数:0.0
-
sp3杂化的碳原子比例:0.8
-
拓扑面积:18.5
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险品标志:Xi
-
储存条件:存放于惰性气体中,并避免接触湿气(以免发生分解)。
SDS
反-2-己烯-1-醇二乙缩醛
模块 1. 化学品
产品名称: trans-2-Hexen-1-al Diethyl Acetal
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第4级
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 可燃液体
造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 远离明火/热表面。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
[储存] 存放于通风良好处。保持凉爽。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
反-2-己烯-1-醇二乙缩醛
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 反-2-己烯-1-醇二乙缩醛
百分比: >93.0%(GC)
CAS编码: 67746-30-9
俗名: 1,1-Diethoxy-trans-2-hexene , Leaf Aldehyde Diethyl Acetal
分子式: C10H20O2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
不适用的灭火剂: 水(有可能扩大灾情。)
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
副危险性的防护措施 移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防爆设备。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离明火和热表面。
采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 使用封闭系统,通风。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
反-2-己烯-1-醇二乙缩醛
模块 8. 接触控制和个体防护
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-极淡的黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 98 °C/4.7kPa
闪点: 65°C
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.85
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
避免接触的条件: 明火
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
反-2-己烯-1-醇二乙缩醛
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: trans-2-Hexen-1-al Diethyl Acetal
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第4级
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 可燃液体
造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 远离明火/热表面。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
[储存] 存放于通风良好处。保持凉爽。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
反-2-己烯-1-醇二乙缩醛
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 反-2-己烯-1-醇二乙缩醛
百分比: >93.0%(GC)
CAS编码: 67746-30-9
俗名: 1,1-Diethoxy-trans-2-hexene , Leaf Aldehyde Diethyl Acetal
分子式: C10H20O2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
不适用的灭火剂: 水(有可能扩大灾情。)
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
副危险性的防护措施 移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防爆设备。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离明火和热表面。
采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 使用封闭系统,通风。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
反-2-己烯-1-醇二乙缩醛
模块 8. 接触控制和个体防护
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-极淡的黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 98 °C/4.7kPa
闪点: 65°C
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.85
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
避免接触的条件: 明火
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
反-2-己烯-1-醇二乙缩醛
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
制备方法与用途
食品添加剂的最大允许使用量及最大允许残留量标准
- 添加剂中文名称: 反式-2-己烯醛二乙缩醛
- 允许使用该种添加剂的食品中文名称: 食品
- 添加剂功能: 食品用香料
- 最大允许使用量(g/kg): 用于配制香精的各香料成分不得超过在GB 2760中的最大允许使用量
- 最大允许残留量(g/kg): 无具体数值,参照GB 2760标准
反应信息
-
作为反应物:描述:反式-2-己烯醛二乙缩醛 在 Bi(1+)*NO3(1-)=BiNO3 作用下, 以 二氯甲烷 为溶剂, 反应 2.0h, 以83%的产率得到2-已烯醛参考文献:名称:一种简单的化学选择性方法,用五水合硝酸铋脱保护乙缩醛和缩酮摘要:DOI:10.1021/jo001202g
-
作为产物:描述:2-已炔 在 [(η5-C5Me5)Ru(η4-MeCH=CHCH=CHCO2H)] triflate 氢气 作用下, 以 氘代甲醇 为溶剂, 24.84 ℃ 、100.0 kPa 条件下, 生成 反式-2-己烯醛二乙缩醛参考文献:名称:In situ 1H-PHIP-NMR studies of the stereoselective hydrogenation of alkynes to ( E)-alkenes catalyzed by a homogeneous [Cp*Ru]+ catalyst摘要:使用[Cp*Ru(烯烃)]+复合物对内炔进行氢化反应,生成(E)-烯烃。这种钌复合物是少数能够直接且选择性地进行反式氢化内炔的均相催化剂之一。我们通过原位PHIP-NMR光谱学(PHIP=邻氢诱导极化)研究了其选择性。利用这种方法,即便在极低的浓度和转化率下,初始生成的产物也能被识别和表征。此外,其后续变化可以高灵敏度和时间分辨率进行评估。采用不同的炔烃底物来展示这种催化剂的广泛适用性。然而,该催化剂在与端炔结合时并不活跃,可能是因为生成了相对稳定的亚乙烯基复合物。为了解释(E)-烯烃的生成,我们提出了一种通过双核复合物的反应机理。DOI:10.1039/b007201j
文献信息
-
Asymmetric simmons-smith reactions using homochiral protecting groups作者:Atsunori Mori、Isao Arai、Hisashi Yamamoto、Hisao Nakai、Yoshinobu AraiDOI:10.1016/s0040-4020(01)88107-2日期:1986.1Asymmetric Simmons-Smith reactions of α,β-unsaturated acetals derived from chiral dialkyl tartrate or (2R,4R)-2,4-pentanediol are described. Treatment of the acetal with diethylzinc and methylene iodide gives a cyclopropane with high diastereoselectivity. The acetal group is readily transformed to the aldehyde or the ester group. In addition, the method is successfully applied to the enantioselective
-
Simple, Mild and Efficient Thioacetalization and Transthioacetalization of Carbonyl Compounds and Deprotection of Thioacetals: Unique Role of Thiols in the Selectivity of Thioacetalization作者:Biswanath Das、Ravirala Ramu、Majjigapu Ravinder Reddy、Gurram MahenderDOI:10.1055/s-2004-834934日期:——Silica supported sodium hydrogen sulfate (NaHSO 4 .SiO 2 ) has been employed for efficient thioacetalization and transthioacetalization of carbonyl compounds in CH 2 Cl 2 at room temperature. Selectivity of thioacetalization was dependent on the thiols used for the conversion. The same catalyst was also found to be effective for deprotection of thioacetals in CH 2 Cl 2 -H 2 O at room temperature.
-
Carbon-carbon bond-forming reactions of zinc homoenolate of esters. A novel three-carbon nucleophile with general synthetic utility作者:Eiichi Nakamura、Satoshi Aoki、Kouichi Sekiya、Hiroji Oshino、Isao KuwajimaDOI:10.1021/ja00260a018日期:1987.12Reactions d'addition sur les composes carbonyles, allylation, arylation, vinylation et acylation en presence ou non de catalyseurs反应 d'addition sur les 组成羰基、烯丙基化、芳基化、乙烯基化和酰化在非脱催化剂的存在下
-
Chemical Library and Structure–Activity Relationships of 11-Demethyl-12-oxo Calanolide A Analogues as Anti-HIV-1 Agents作者:Tao Ma、Li Liu、Hai Xue、Li Li、Chunyan Han、Lin Wang、Zhiwei Chen、Gang LiuDOI:10.1021/jm701405p日期:2008.3.13(+)-Calanolide A (1) as a natural product was previously found as an inhibitor of HIV-1 reverse tratiscriptase. In our further investigation of its template, racemic 11-demethyl-12-oxo calanolide A (15), which had two fewer chiral carbon centers at the C-11 and C-12 positions than (+)-calanolide A, had a comparably inhibitory activity and better therapeutic index (EC(50) = 0.11 mu M, TI = 818) against HIV-1 in vitro. A library based on its structural core was then designed and synthesized with introduction of nine diversity points in this article. The evaluations of anti-HIV-1 activity in vitro concluded their structure-activity relationships (SARs). A novel compound (10-bromomethyl-11-demethyl-12-oxo calanolide A, 123) was identified to have much higher inhibitory potency and therapeutic index (EC(50) = 2.85 nM, TI > 10,526) than those of the class compound against HIV-1. This finding provided a very important clue that modifications of the C ring at the C-10 position may be conducted to obtain drug candidates with better activity against HIV-1.(+)-Calanolide A (1) 作为一种天然产物,先前被发现是HIV-1逆转录酶的抑制剂。在我们对其模板的进一步研究中,外消旋的11-去甲基-12-酮基calanolide A (15) 被发现。与(+)-calanolide A相比,它在C-11和C-12位上少两个手性碳中心,但其对HIV-1的抑制活性相当,并且具有更好的治疗指数(EC(50) = 0.11 μM,TI = 818)。随后,基于其结构核心设计并合成了一个化合物库,并在本文中引入了九个多样性点。体外评估抗HIV-1活性得出了它们的结构-活性关系(SARs)。发现了一个新的化合物(10-溴甲基-11-去甲基-12-酮基calanolide A,123),其对HIV-1的抑制效力和治疗指数(EC(50) = 2.85 nM,TI > 10,526)显著高于该类化合物。这一发现提供了非常重要的线索,即对C环的C-10位进行修饰,可能会得到对HIV-1活性更好的药物候选物。
-
Oxathioacetalization, thioacetalization and transthioacetalization of carbonyl compounds by N-bromosuccinimide: selectivity and scope作者:Ahmed Kamal、Gagan Chouhan、Kaleem AhmedDOI:10.1016/s0040-4039(02)01610-6日期:2002.9Efficient oxathioacetalization, thioacetalization and transthioacetalization of carbonyl compounds have been achieved in high yields employing N-bromosuccinimide as a catalyst.
表征谱图
-
氢谱1HNMR
-
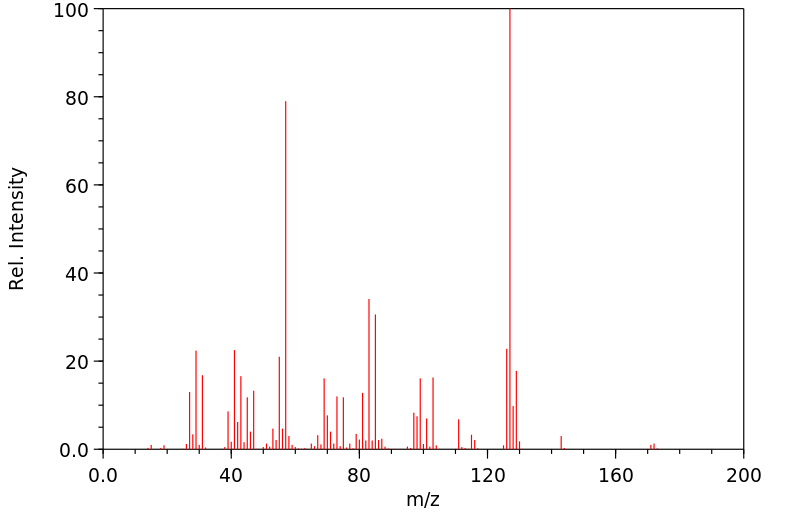
质谱MS
-
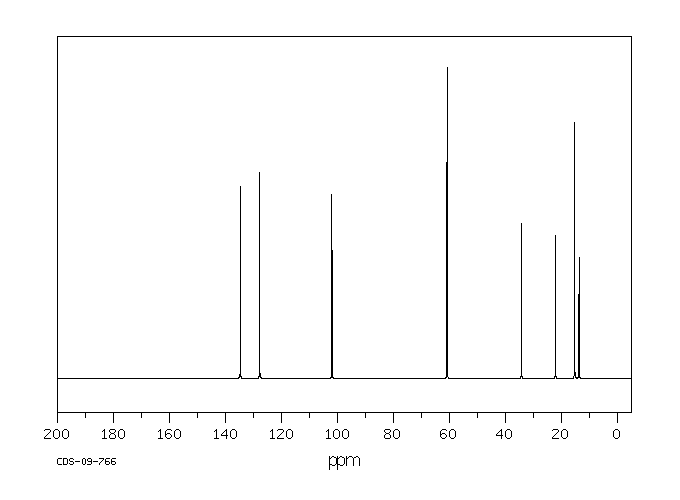
碳谱13CNMR
-
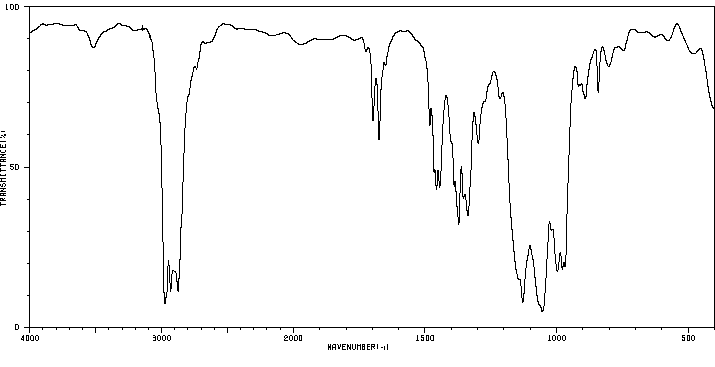
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷