四正丁基锗 | 1067-42-1
中文名称
四正丁基锗
中文别名
四丁基锗
英文名称
tetrabutylgermanium
英文别名
Tetrabutyl-german;Tetra-n-butylgerman;Tetra-n-butyl-germanium;Tetrabutylgermane
CAS
1067-42-1
化学式
C16H36Ge
mdl
——
分子量
301.052
InChiKey
HDVLQIDIYKIVRE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-74.1°C
-
沸点:>100 °C(lit.)
-
密度:0.93 g/mL at 25 °C(lit.)
-
闪点:>230 °F
-
介电常数:2.3300000000000001
-
稳定性/保质期:
在常温常压下稳定,避免与酸接触以防止氧化。
计算性质
-
辛醇/水分配系数(LogP):5.37
-
重原子数:17
-
可旋转键数:12
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
TSCA:Yes
-
危险品标志:Xn
-
安全说明:S36
-
危险类别码:R20/21/22
-
WGK Germany:3
-
海关编码:2931900090
-
储存条件:请将容器密封保存,并存放在阴凉、干燥处。
SDS
| Name: | Tetrabutyl germanium Material Safety Data Sheet |
| Synonym: | |
| CAS: | 1067-42-1 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 1067-42-1 | Tetrabutyl germanium | 213-929-3 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up or absorb material, then place into a suitable clean, dry, closed container for disposal. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use with adequate ventilation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed.
Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 1067-42-1: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: colorless
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: > 100 deg C
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: > 110 deg C (> 230.00 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: .9300g/cm3
Molecular Formula: CH3(CH2)3!4Ge
Molecular Weight: 301.06
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Irritating and toxic fumes and gases.
Hazardous Polymerization: Not available.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 1067-42-1 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Tetrabutyl germanium - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing group:
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 1067-42-1: No information available.
Canada
CAS# 1067-42-1 is listed on Canada's NDSL List.
CAS# 1067-42-1 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 1067-42-1 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— tributylgermanium hydride 998-39-0 C12H28Ge 244.944
反应信息
-
作为反应物:参考文献:名称:Clark, K. Brady; Griller, David, Organometallics, 1991, vol. 10, # 3, p. 746 - 750摘要:DOI:
-
作为产物:参考文献:名称:Redistributions in Halogenosilanes and Alkylhalogenosilanes; Alkyliodochlorosilanes and Dimethylisocyanatochlorosilane摘要:DOI:10.1021/ja01156a098
文献信息
-
Tributylgermanium hydride as a replacement for tributyltin hydride in radical reactionsElectronic supplementary information (ESI) available: full experimental details and data. See http://www.rsc.org/suppdata/ob/b3/b310520b/作者:W. Russell Bowman、Sussie L. Krintel、Mark B. SchillingDOI:10.1039/b310520b日期:——Tributylgermanium hydride (Bu(3)GeH) can be used as an alternative to tributyltin hydride (Bu(3)SnH) as a radical generating reagent with a wide range of radical substrates. Tributylgermanium hydride has several practical advantages over tributyltin hydride, e.g. low toxicity, good stability and much easier work-up of reactions. The reagent can be easily prepared in good yield and stored indefinitely氢化三丁基锗(Bu(3)GeH)可以用作氢化三丁基锡(Bu(3)SnH)的替代物,用作具有多种自由基底物的自由基发生剂。氢化三丁基锗比氢化三丁基锡具有多个实用优势,例如低毒性,良好的稳定性和更容易进行的反应后处理。该试剂可以容易地以高收率制备并且可以无限期地存储。合适的底物包括碘化物,溴化物,活化的氯化物,硒化苯基,叔硝基烷烃,硫代羰基咪唑啉化物和巴顿酯。烷基,乙烯基和芳基自由基可以在包括还原和环化过程在内的自由基反应中生成。可以使用常见的自由基引发剂,例如ACCN和三乙基硼烷。与Bu(3)SnH相比,Bu(3)GeH的碳中心自由基吸收氢的速度较慢,这有助于提高环化收率。苯硫醇的极性反转催化(PRC)可用于产生稳定的自由基中间体的反应,该中间体不会从Bu(3)GeH中提取氢。
-
Novel alkylation of aromatic nitriles via photo-induced electron transfer of group 14 metal-carbon σ donors作者:Soichiro Kyushin、Yukihiro Masuda、Kazuhiro Matsushita、Yasuhiro Nakadaira、Mamoru OhashiDOI:10.1016/s0040-4039(00)97074-6日期:——Photo-induced electron transfer reactions of tetraalkylsilanes, -germanes, and -stannanes with aromatic nitriles afforded alkylated products. The mechanism was investigated by use of a radical clock.
-
IR Spectra of <i>n</i>-Bu<sub>4</sub>M (M = Si, Ge, Sn, Pb), <i>n</i>-BuAuPPh<sub>3</sub>-<i>d</i><sub>15</sub>, and “<i>n</i>-Bu” on a Gold Surface作者:Jiří Kaleta、Lucie Bednárová、Martina Čížková、Jin Wen、Eva Kaletová、Josef MichlDOI:10.1021/acs.jpca.7b03404日期:2017.6.22and DFT-calculated IR spectra of n-Bu4M (M = Si, Ge, Sn, Pb), (CH3CH2CH213CD2)4Sn, and n-BuAuPPh3-d15 are reported and assigned. The asymmetric CH stretching vibration of the CH2 group adjacent to the metal atom appears as a distinct shoulder at ∼2934 cm–1, whereas for other CH2 groups it is located at ∼2922 cm–1. The characteristic peak at ∼2899 cm–1 is attributed to an overtone of a symmetric CH2 bend报告了n -Bu 4 M(M = Si,Ge,Sn,Pb),(CH 3 CH 2 CH 2 13 CD 2)4 Sn和n -BuAuPPh 3 - d 15的观察值和DFT计算的IR谱,并且已分配。与金属原子相邻的CH 2基团的不对称CH拉伸振动在〜2934 cm –1处表现为明显的肩峰,而对于其他CH 2基团,其位于〜2922 cm –1处。在〜2899 cm –1处的特征峰归因于对称CH 2的泛音在〜1445 cm –1处弯曲。在n -BuAuPPh 3 - d 15中,丁基的CH拉伸振动向较低的频率偏移约10 cm –1,并提供了两种可能的合理化方法。
-
Donor-acceptor complexes of organometals and iodine. Alkyl ligands as probes for steric effects in charge transfer作者:S. Fukuzumi、J. K. KochiDOI:10.1021/j100443a009日期:1980.3
-
Reactivity of dianionic hexacoordinate germanium complexes toward organometallic reagents. A new route to organogermanes作者:G. Cerveau、C. Chuit、R. J. P. Corriu、C. ReyeDOI:10.1021/om00051a049日期:1991.5Lithium and potassium tris(benzene-1,2-diolato)germanates (2a and 2b, respectively) and potassium tris(butane-2,3-diolato)germanate (3) are easily prepared from GeO2 in quantitative yields. They are very reactive toward organometallic reagents, the reactivity depending on the ligands on the germanium. Complexes 2 react with an excess of Grignard reagent to give the corresponding tetraorganogermanes R4Ge while the less reactive complex 3 leads to the functional triorganogermanes R3GeX. Tetraorganogermanes can also be prepared from complex 2b by reaction with organic bromides in the presence of Mg (Barbier reaction). The influence of Cp2TiCl2 and MgBr2 on the reactivity of Grignard reagents with these complexes was also investigated: in both cases formation of triorganogermanes was favored.
表征谱图
-
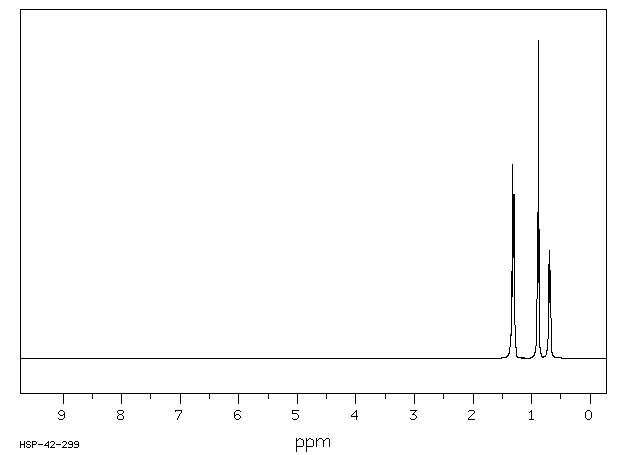
氢谱1HNMR
-
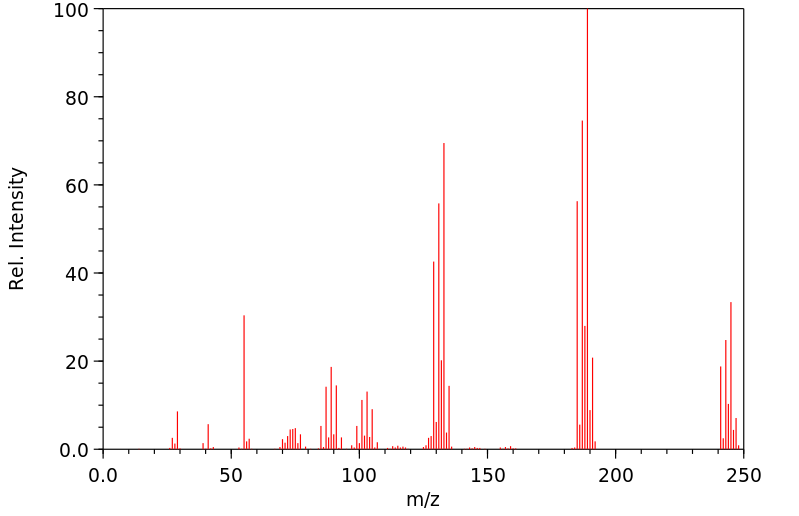
质谱MS
-
碳谱13CNMR
-
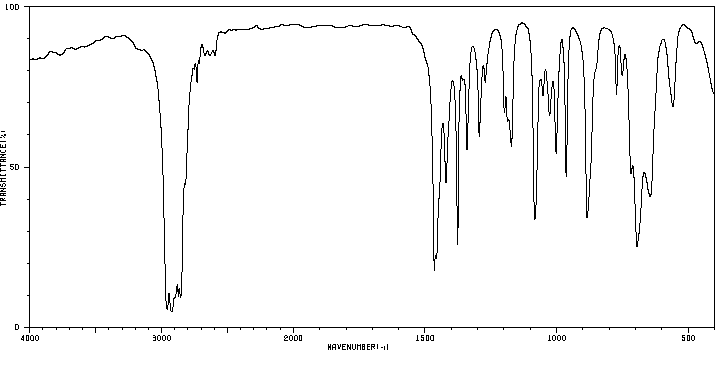
红外IR
-
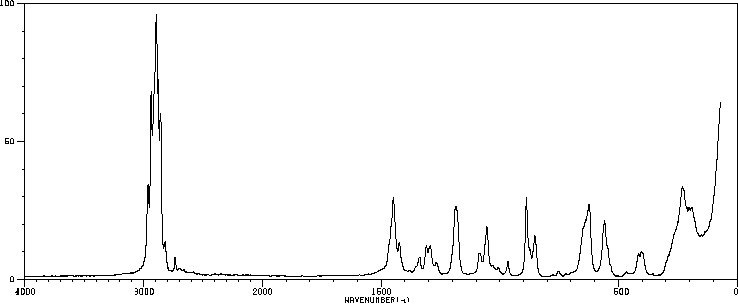
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2-溴乙氧基)-特丁基二甲基硅烷
鲸蜡基聚二甲基硅氧烷
高纯二甲基镉
骨化醇杂质DCP
马沙骨化醇中间体
马来酸双(三甲硅烷)酯
顺式-二氯二(二甲基硒醚)铂(II)
顺-N-(1-(2-乙氧基乙基)-3-甲基-4-哌啶基)-N-苯基苯酰胺
降钙素杂质13
降冰片烯基乙基三甲氧基硅烷
降冰片烯基乙基-POSS
间-氨基苯基三甲氧基硅烷
镓,碘二甲基-
镓,二(1,1-二甲基乙基)甲基-
镁,溴[(1E)-1-甲基-1-丙烯基]-
镁,溴[(1E)-1-甲基-1-丙烯基]-
镁,氯[[二甲基(1-甲基乙氧基)甲硅烷基]甲基]-
锰,五羰基(三甲基甲锡烷基)-,(OC-6-22)-
锡烷,乙氧基三甲基-
锡烷,三丁基(1E)-1-庚烯基-
锡烷,三丁基(1-甲基-2-丁烯基)-,(Z)-
锡烷,三(1,1-二甲基乙基)乙炔基-
锡烷,[4-(2-乙基己基)-2-噻吩基]三甲基-
锡烷,(4-氯二环[2.2.1]庚-1-基)三甲基-
锡烷,(1E)-1-丁烯-3-炔基三丁基-
锡杂苯
锑杂苯
锑,二溴三丁基-
锌,溴-1-己炔基-
铷,[三(三甲基甲硅烷基)甲基]-
铝,三庚基-
铝,丁氧基二(2-甲基丙基)-
铅烷,三丁基-1-己炔基-
铂(0)-1,3-二乙烯-1,1,3,3-四甲基二硅氧烷
钾(4-{[二甲基(2-甲基-2-丙基)硅烷基]氧基}-1-丁炔-1-基)(三氟)硼酸酯(1-)
钼,亚甲基-
金刚烷基乙基三氯硅烷
酰氧基丙基双封头
达格列净杂质
辛醛,8-[[(1,1-二甲基乙基)二甲基甲硅烷基]氧代]-
辛甲基-1,4-二氧杂-2,3,5,6-四硅杂环己烷
辛基锡
辛基铵甲烷砷酸盐
辛基衍生化硅胶(C8)ZORBAX?LP100/40C8
辛基硅三醇
辛基甲基二乙氧基硅烷
辛基氧代锡烷
辛基三甲氧基硅烷
辛基三氯硅烷
辛基(三苯基)硅烷