安他唑啉 | 91-75-8
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:120-122°
-
沸点:398.57°C (rough estimate)
-
密度:0.9786 (rough estimate)
-
物理描述:Solid
-
颜色/状态:White, crystalline powder
-
气味:Odorless
-
味道:Bitter
-
溶解度:663 mg/L (at 30 °C)
-
蒸汽压力:2.3X10-8 mm Hg at 25 °C (est)
-
分解:When heated to decomposition it emits toxic fumes of /nitrogen oxides/.
-
解离常数:pKa = 4.90 (non-cyclic tertiary nitrogen); pKa = 10.68 (tertiary imidazoline nitrogen) (est)
-
保留指数:2280;2315;2295;2320;2330;2328
计算性质
-
辛醇/水分配系数(LogP):2.6
-
重原子数:20
-
可旋转键数:5
-
环数:3.0
-
sp3杂化的碳原子比例:0.235
-
拓扑面积:27.6
-
氢给体数:1
-
氢受体数:2
ADMET
安全信息
-
安全说明:S22,S24/25
-
储存条件:-20°C
SDS
制备方法与用途
盐酸安他唑啉(4,5-二氮-N-苯甲基-1H-咪唑-2-甲胺盐酸盐)是一种乙二胺类组胺受体拮抗剂,具有抗组胺和抗胆碱作用。它能干扰心肌细胞膜对钾、钠等离子的渗透,从而减缓心肌纤维间的传导速度。临床上主要用于治疗室性早搏以及洋地黄毒苷引起的心动过速,属IA类抗心律失常药。目前关于盐酸安他唑啉的研究侧重于其临床应用的安全性和有效性,以及采用光谱法和色谱法测定制剂中药物含量,但有关生物样品中的药物浓度测定及药代动力学研究尚未见报道。
临床研究盐酸安他唑啉(安他唑啉)是一种治疗多种心律失常的药物。本研究旨在观察其在不伴有明显器质性心脏病患者中的安全性和有效性。采用双盲、随机、阳性对照试验,对照药物为稳心颗粒。共入选60例不伴有明显器质性心脏病的室性早搏(简称室早)患者,并于停用其他抗心律失常药2周后随机分为安他唑啉治疗组和稳心颗粒治疗组。服药治疗第2周进行随访记录不良反应等临床资料,满4周后复查Holter。疗效结果为对比治疗前后24小时室早的数目;安全性指标则包括治疗前后患者的生化检查结果。
共入选72例患者,其中男性37例(51.39%),平均年龄(45.06±12.33)岁。实际完成随访67例(研究组34例)。两组间基本临床资料匹配。治疗4周后,安他唑啉治疗组与稳心颗粒治疗组24小时室早数目均有明显降低,分别为70.11%和67.80%,但两者之间没有统计学差异(P=0.35)。此外,研究组中有88.24%的患者24小时室早减少超过75%,对照组为78.79%,同样未见显著性差异(P=0.61)。两组均无严重不良反应。结论认为,在临床实践中,安他唑啉对于治疗一些不合并严重心脏疾病的室性早搏具有较好的有效性和较高的安全性。
生物活性 ArgirelineArgireline是一种新型的神经肽,广泛应用于局部皮肤护理。
化学性质盐酸安他唑啉为白色结晶性粉末,熔点237-241℃。它溶于乙醇,略溶于水,不溶于氯仿、乙醚和苯。
用途盐酸安他唑啉作为抗心律失常药物,用于治疗房性和室性早搏、阵发性心动过速等。此外,也可用作抗组织胺药物,适用于过敏性疾病。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— N-benzyl-N-phenyl-thioglycine amide 409111-05-3 C15H16N2S 256.371 (苄基苯基氨基)乙腈 2-(benzyl(phenyl)amino)acetonitrile 36271-19-9 C15H14N2 222.29 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 N-(2-氨基乙基)-2-[苯基(苯基甲基)氨基]乙酰胺 N-<(N-benzylanilino)acetyl>ethylenediamine 26953-37-7 C17H21N3O 283.373 N-苄基-N-苯基乙酰胺 N-phenyl-N-benzylacetamide 6840-29-5 C15H15NO 225.29
反应信息
-
作为反应物:参考文献:名称:Charge-Transfer-Complexes, 2nd Mitt. 具有咪唑啉部分结构的药物的分子复合物和自由基形成摘要:根据 UV/Vis、IR、FIR、1H NMR 和 13C NMR 数据,tolazoline (1)、naphazoline (2)、xylometazoline (3)、oxymetazoline (4) 和antazoline (5) 与碘形成电荷转移络合物 (CT) ) 的结构 6, 7 和 8 来自, 而 7,7,8,8-四氰基醌二甲烷 (TCNQ), 2,3-二氯-5,6-二氰基-对苯醌 (DDQ), 对氯苯醌, 2, 4,5,7-四硝基芴酮(TeNF)和9-二氰基芴酮-2,4,7-三硝基芴酮(DTF)主要是电子供体受体复合物(EDA)和自由基阴离子(Scheme 3-8)之间的溶剂依赖性平衡产生的。结果被转移到 1-5 的直流检测,并用于 Priscol® 片剂和 Otriven® 滴剂的质量控制。DOI:10.1002/ardp.19843170311
-
作为产物:描述:参考文献:名称:The Preparation and Characterization of New Antazoline Salts with Dicarboxylic Acids摘要:New antazoline salts with organic acids (fumaric acid, oxalic acid, and maleic acid) were prepared. The effect of the crystallization solvent and mechanochemical treatment on the crystalline forms of these salts was studied. Two polymorphs of antazoline hydrogen maleate were identified and their relative stability was determined. The molecular structures of antazoline hydrogen oxalate and antazoline hydrogen maleate showed differences in antazoline cation conformation. In crystal structures of all salts both imidazoline nitrogens of antazoline cation are involved in hydrogen bond formation with carboxyl groups of the acid.DOI:10.1080/15421406.2014.905041
文献信息
-
[EN] 3,5-DIAMINO-6-CHLORO-N-(N-(4-PHENYLBUTYL)CARBAMIMIDOYL) PYRAZINE-2- CARBOXAMIDE COMPOUNDS<br/>[FR] COMPOSÉS 3,5-DIAMINO -6-CHLORO-N-(N- (4-PHÉNYLBUTYL)CARBAMIMIDOYL) PYRAZINE-2-CARBOXAMIDE申请人:PARION SCIENCES INC公开号:WO2014099673A1公开(公告)日:2014-06-26The present invention relates compounds of the formula: or pharmaceutically acceptable salts thereof, useful as sodium channel blockers, as well as compositions containing the same, processes for the preparation of the same, and therapeutic methods of use therefore in promoting hydration of mucosal surfaces and the treatment of diseases including cystic fibrosis, chronic obstructive pulmonary disease, asthma, bronchiectasis, acute and chronic bronchitis, emphysema, and pneumonia.
-
CHLORO-PYRAZINE CARBOXAMIDE DERIVATIVES WITH EPITHELIAL SODIUM CHANNEL BLOCKING ACTIVITY申请人:Parion Sciences, Inc.公开号:US20140171447A1公开(公告)日:2014-06-19This invention provides compounds of the formula I: and their pharmaceutically acceptable salts, useful as sodium channel blockers, compositions containing the same, therapeutic methods and uses for the same and processes for preparing the same.这项发明提供了式I的化合物及其药用盐,可用作钠通道阻滞剂,包含这些化合物的组合物,以及用于这些化合物的治疗方法和用途,以及制备这些化合物的方法。
-
3-Aminocyclopentanecarboxamides as modulators of chemokine receptors申请人:Xue Chu-Biao公开号:US20060004018A1公开(公告)日:2006-01-05The present invention is directed to compounds of Formula I: which are modulators of chemokine receptors. The compounds of the invention, and compositions thereof, are useful in the treatment of diseases related to chemokine receptor expression and/or activity.
-
CYCLOPROPYLAMINES AS LSD1 INHIBITORS
-
Benzoxazinyl-amidocyclopentyl-heterocyclic modulators of chemokine receptors申请人:Goble D. Stephen公开号:US20070238723A1公开(公告)日:2007-10-11Cyclopentyl compounds linked to a benzoxazinyl group through an amido moiety utilizing the ring nitrogen of the benzoxazine, and further substituted with a heterocyclic moiety, such compounds represented by formula I: which are used to modulate the CCR-2 chemokine receptor to prevent or treat inflammatory and immunoregulatory disorders and diseases, allergic diseases, atopic conditions including allergic rhinitis, dermatitis, conjunctivitis, and asthma, as well as autoimmune pathologies such as rheumatoid arthritis and atherosclerosis; and pharmaceutical compositions comprising these compounds and the use of these compounds and compositions.
表征谱图
-
氢谱1HNMR
-
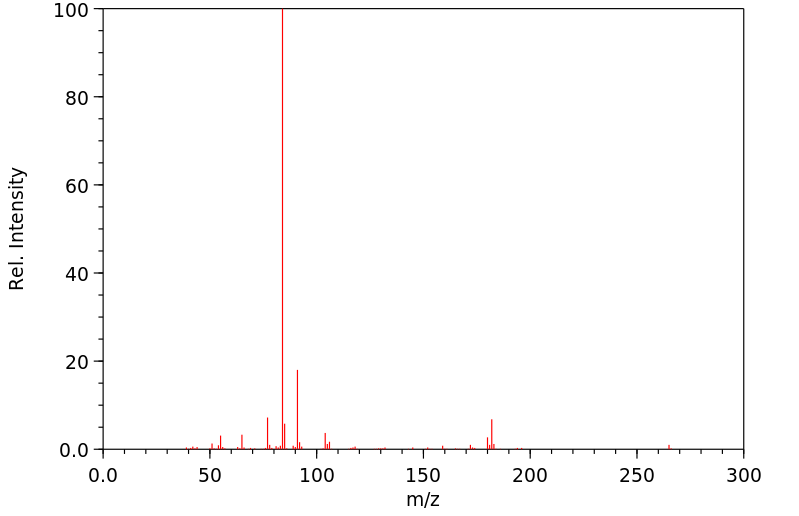
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息