(4E)-4-十二碳烯 | 7206-15-7
中文名称
(4E)-4-十二碳烯
中文别名
——
英文名称
trans-4-dodecene
英文别名
dodec-4t-ene;4-dodecene;trans-Dodec-4-en;(E)-dodec-4-ene
CAS
7206-15-7
化学式
C12H24
mdl
——
分子量
168.323
InChiKey
PHCKFVVLVZFFLU-VQHVLOKHSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-38.4°C (estimate)
-
沸点:207.3°C (estimate)
-
密度:0.7761 (estimate)
-
保留指数:1190;1190;1181
计算性质
-
辛醇/水分配系数(LogP):5.8
-
重原子数:12
-
可旋转键数:8
-
环数:0.0
-
sp3杂化的碳原子比例:0.83
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
反应信息
-
作为反应物:描述:(4E)-4-十二碳烯 在 [OsIII(OH)(H2O)(N,N’-dimethyl-2,11-diaza-[3.3](2,6)pyridinophane)](PF6)2 、 双氧水 作用下, 以 水 、 正丁醇 为溶剂, 反应 5.0h, 以53%的产率得到(4R,5R)-dodecane-4,5-diol参考文献:名称:带有大环配体的(III)和O(V)配合物:烯烃与过氧化氢的顺式-二羟基化反应的简单高效催化体系摘要:一个简单的协议,它使用[Os III(OH)(H 2 O)(L -N 4 Me 2)](PF 6)2(1 ; L- N 4 Me 2 = N,N'-二甲基-2,11 ‐diaza [3.3](2,6)pyridinophane)作为催化剂,H 2 O 2作为有效顺式的末端氧化剂介绍了烯烃的1,2-二羟基化反应。含有吸电子基团的未官能化(或脂肪族)烯烃和烯烃/苯乙烯被选择性氧化成相应的邻位二醇,收率良好至极佳(46–99%)。在催化反应中,烯烃:H 2 O 2的化学计量为1:1,因此氧化效率非常高。对于环己烯的二羟基化,可以将1的催化量降低至0.01 mol%,以实现5500的极高周转率。活性氧化剂被鉴定为Os V(O)(OH)种类(2)。通过氢过氧化物加合物形成的Os III(OOH)物种。活性氧化剂2 已成功分离并进行了晶体学表征。DOI:10.1002/asia.201300329
-
作为产物:参考文献:名称:碱金属将乙炔化合物还原为 (E)-烯烃 - 范围调查摘要:已经开发了用于通过碱金属立体定向还原长碳链二取代乙炔的有效程序。含有两个或多个(分离的)三键的乙炔比单炔更容易还原。DOI:10.1002/(sici)1099-0690(199904)1999:4<775::aid-ejoc775>3.0.co;2-3
文献信息
-
Asymmetric Allylic Alkylation of Acyclic Allylic Ethers with Organolithium Reagents作者:Manuel Pérez、Martín Fañanás-Mastral、Valentín Hornillos、Alena Rudolph、Pieter H. Bos、Syuzanna R. Harutyunyan、Ben L. FeringaDOI:10.1002/chem.201202251日期:2012.9.17CuI/phosphoramidite‐catalyzed asymmetric allylic alkylation of allyl ethers with organolithium reagents is reported (see scheme). The use of organolithium reagents is essential for this catalytic CC bond formation due to their compatibility with different Lewis acids. The versatility of allylic ethers under the copper‐catalyzed reaction conditions with organolithium reagents is demonstrated in the shortest
-
Le Bigot, Y.; Delmas, M.; Gaset, A., Synthetic Communications, 1982, vol. 12, # 2, p. 107 - 112作者:Le Bigot, Y.、Delmas, M.、Gaset, A.DOI:——日期:——
-
KATRITZKY, A. R.;EL-MOWAFY, AZZAHRA, M., J. ORG. CHEM., 1982, 47, N 18, 3506-3511作者:KATRITZKY, A. R.、EL-MOWAFY, AZZAHRA, M.DOI:——日期:——
-
LE, BIGOT YVES;DELMAS, MICHEL;GASET, ANTOINE, TETRAHEDRON, 44,(1988) N 4, 1057-1072作者:LE, BIGOT YVES、DELMAS, MICHEL、GASET, ANTOINEDOI:——日期:——
表征谱图
-
氢谱1HNMR
-
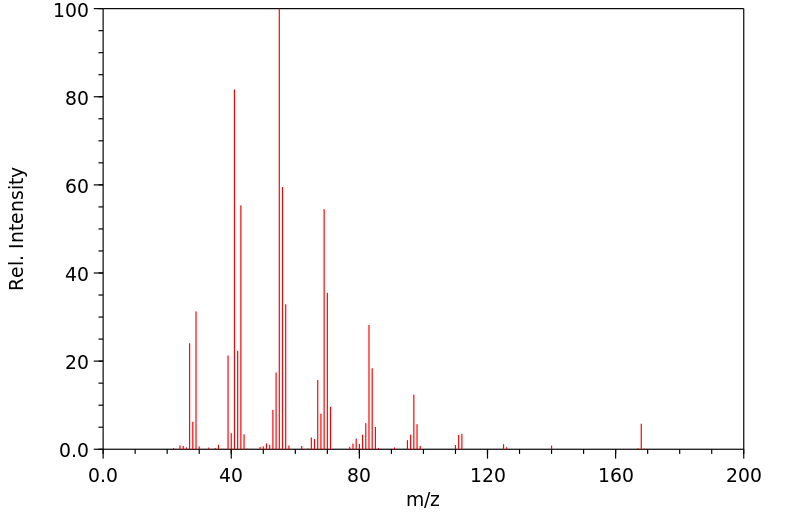
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-