(5beta)-3-氧代-胆烷-24-酸 | 1553-56-6
分子结构分类
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:140-141 °C
-
沸点:509.3±23.0 °C(Predicted)
-
密度:1.069±0.06 g/cm3(Predicted)
-
溶解度:可溶于氯仿(少许)、甲醇(少许)
-
碰撞截面:205.5 Ų [M-H]- [CCS Type: DT, Method: single field calibrated]
计算性质
-
辛醇/水分配系数(LogP):5.8
-
重原子数:27
-
可旋转键数:4
-
环数:4.0
-
sp3杂化的碳原子比例:0.916
-
拓扑面积:54.4
-
氢给体数:1
-
氢受体数:3
安全信息
-
储存条件:室温
SDS
: 5β-Cholanic acid-3-one
产品名称
1.2 鉴别的其他方法
3-Keto-5β-cholanic acid
Dehydrolithocholic acid
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
根据化学品全球统一分类与标签制度(GHS)的规定,不是危险物质或混合物。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: 3-Keto-5β-cholanic acid
别名
Dehydrolithocholic acid
: C24H38O3
分子式
: 374.56 g/mol
分子量
无
模块 4. 急救措施
4.1 必要的急救措施描述
吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。
皮肤接触
用肥皂和大量的水冲洗。
眼睛接触
用水冲洗眼睛作为预防措施。
食入
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。
4.2 主要症状和影响,急性和迟发效应
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
防止粉尘的生成。 防止吸入蒸汽、气雾或气体。
6.2 环境保护措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
扫掉和铲掉。 存放进适当的闭口容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
常规的工业卫生操作。
个体防护设备
眼/面保护
请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
根据危险物质的类型,浓度和量,以及特定的工作场所来选择人体保护措施。,
防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
不需要保护呼吸。如需防护粉尘损害,请使用N95型(US)或P1型(EN 143)防尘面具。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 固体
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
无数据资料
f) 起始沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸汽压
无数据资料
l) 蒸汽密度
无数据资料
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 应避免的条件
无数据资料
10.5 不兼容的材料
强酸, 强碱
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 如果通过皮肤吸收可能是有害的。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: FZ2281400
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 潜在的生物蓄积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。
受污染的容器和包装
作为未用过的产品弃置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
3-氧代-5β-胆固醇酸(脱氢胆酸)是一种胆汁酸代谢产物,能够通过直接与关键转录因子RORγt结合(Kd=1.13 μM),抑制TH17细胞分化。
体外研究使用3-氧代-5β-胆固醇酸处理显著减少了RORγt报告基因的活性。这些数据表明,3-氧代-5β-胆固醇酸可能通过物理结合与RORγt,并抑制其转录活性来抑制TH17细胞分化。
体内研究0.3% (w/w) 的3-氧代-5β-胆固醇酸(脱氢胆酸)灌胃给药一周显著减少了回肠中TH17细胞的百分比。
| 动物模型 | B6 Jax小鼠(带有含SFB的粪便悬浮液) |
|---|---|
| 剂量 | 0.3% (w/w) |
| 给药方式 | 灌胃,给药一周 |
| 结果 | 显著减少了回肠中TH17细胞的百分比 |
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 去氢胆酸 dehydrocholic acid 81-23-2 C24H34O5 402.531 —— methyl 3,7,12-trioxo-5β-cholan-24-oate 7727-82-4 C25H36O5 416.558 石胆酸 Lithocholic acid 434-13-9 C24H40O3 376.58 异石胆酸 Isolithocholic acid 1534-35-6 C24H40O3 376.58 3-Alpha-羟基-5-beta-24-胆烷酸甲酯 methyl lithocholate 1249-75-8 C25H42O3 390.607 胆酸 cholate 81-25-4 C24H40O5 408.579 —— 5β-cholane-3α,24-diol —— C24H42O2 362.596 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 甲基-3-氧代-5beta-胆烷-24-酸酯 methyl 3-oxo-5β-cholan-24-oate 1173-32-6 C25H40O3 388.591 5β-胆烷酸 cholanic acid 546-18-9 C24H40O2 360.58 —— methyl 5β-cholan-24-oate 2204-14-0 C25H42O2 374.607 石胆酸 Lithocholic acid 434-13-9 C24H40O3 376.58 异石胆酸 Isolithocholic acid 1534-35-6 C24H40O3 376.58 —— 26.27-Bis-nor-5β-cholestan-24-on 14949-16-7 C25H42O 358.608 —— 3β-amino-5β-cholan-24-oic acid 106526-69-6 C24H41NO2 375.595 —— 3α-amino-5β-cholan-24-oic acid 106697-48-7 C24H41NO2 375.595 异胆酸甲酯-d7 methyl 3β-hydroxy-5β-cholan-24-oate 5405-42-5 C25H42O3 390.607 5-Beta-卓兰-24-醇 5β-cholan-24-ol 3110-99-4 C24H42O 346.597 —— 3-oxo-5β-cholan-24-oic acid (cholan-24-oic acid methyl ester)-3-yl ester, (3α,5β) 668989-83-1 C49H78O5 747.155 —— 3α-amino-5β-colanato di metile 106697-49-8 C25H43NO2 389.622 —— 3β-aminolithocholic acid methyl ester 106697-50-1 C25H43NO2 389.622 —— 3-oxo-5β-chol-1-en-24-oic acid 1452-30-8 C24H36O3 372.548 (1beta,3alpha,5beta)-1,3-二羟基-胆烷-24-酸 1β,3α-dihydroxy-5β-cholan-24-oic acid 89238-74-4 C24H40O4 392.579 —— 5β cholene-1, one-3, oate-24 de methyle 91667-86-6 C25H38O3 386.575 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:胆汁酸衍生物的微波辅助绿色合成及糖皮质激素受体结合评价摘要:在此,我们展示了在水介质中存在 Oxone® 的情况下,微波辅助 AlCl 3催化的胆汁酸羟基氧化。观察到 Wolff-Kishner 将合成的胆汁酸氧代衍生物还原为 5β-胆酸的速率显着提高。最简单的胆汁酸的酰胺化反应和脱氧胆酸的氨解反应是在没有溶剂和催化剂的情况下在密封容器微波条件下完成的。因为据报道 5β-胆酸在帕金森病细胞模型中调节糖皮质激素受体信号传导,我们在体外测试了 5β-胆酸和脱氧胆酸衍生物对糖皮质激素受体的亲和力使用基于酵母的荧光屏幕。用泼尼松龙处理表达 GR 的酵母导致荧光的剂量依赖性增加;而 5β-胆酸以更温和的亲和力与糖皮质激素受体结合。同样,分子对接也表明 5β-胆酸可以与糖皮质激素受体结合,与已知的 GR 配体具有相似的几何形状。DOI:10.1039/d0md00311e
-
作为产物:描述:参考文献:名称:Emerging Options in the Treatment of Bipolar Disorders摘要:双相情感障碍是一种常见且严重的疾病,其临床结果通常不尽如人意。目前针对该疾病的治疗选择较少。越来越多的新型治疗选项正在研究中,包括抗癫痫药、非典型抗精神病药,以及如omega-3脂肪酸和经颅磁刺激等选项。在抗癫痫药中,拉莫三嗪目前是积累了最多对照临床数据的药物,特别是在抑郁和快速循环发作阶段。奥氮平目前是针对狂躁症有最多证据的非典型抗精神病药物,尽管关于其他非典型抗精神病药物如利培酮和齐拉西酮的数据也在逐渐出现。针对该疾病其他阶段非典型药物的数据尚待发布。随着新药物的出现,治疗这一难治性疾病的选择正在增加。DOI:10.2165/00003495-200161100-00004
文献信息
-
Synthesis and Anticancer Activity of Hybrid Molecules Based on Lithocholic and (5Z,9Z)-Tetradeca-5,9-dienedioic Acids Linked via Mono(di,tri,tetra)ethylene Glycol and α,ω-Diaminoalkane Units作者:Vladimir A. D’yakonov、Regina A. Tuktarova、Lilya U. Dzhemileva、Svetlana R. Ishmukhametova、Usein M. DzhemilevDOI:10.3390/ph14020084日期:——
For the first time, hybrid molecules were synthesized on the basis of lithocholic and (5Z,9Z)-1,14-tetradeca-5,9-dienedicarboxylic acids, obtained in two stages using the homo-cyclomagnesiation reaction of 2-(hepta-5,6-diene-1-yloxy)tetrahydro-2H-pyran at the key stage. The resulting hybrid molecules containing 5Z,9Z-dienoic acids are of interest as novel synthetic biologically active precursors to create modern drugs for the treatment of human oncological diseases. The synthesized hybrid molecules were found to exhibit extremely high in vitro inhibitory activity against human topoisomerase I, which is 2–4 times higher than that of camptothecin, a known topoisomerase I inhibitor. Using flow cytometry and fluorescence microscopy, it was first shown that these new molecules are efficient apoptosis inducers in HeLa, U937, Jurkat, K562, and Hek293 cell cultures. In addition, the results of investigations into the effect of the synthesized acids on mitochondria and studies of possible DNA damage in Jurkat tumor cells are also presented.
首次,基于麻黄酸和(5Z,9Z)-1,14-十四碳-5,9-二烯二羧酸,在两个阶段使用2-(庚-5,6-二烯-1-氧基)四氢-2H-吡喃的同环合镁反应合成了杂化分子。含有5Z,9Z-二烯酸的这些杂化分子作为新型合成生物活性前体,对于开发治疗人类肿瘤疾病的现代药物具有重要意义。合成的杂化分子显示出极高的体外抑制人类拓扑异构酶I活性,比已知的拓扑异构酶I抑制剂喜树碱高2-4倍。首次利用流式细胞术和荧光显微镜观察到这些新分子在HeLa、U937、Jurkat、K562和Hek293细胞培养中是有效的凋亡诱导剂。此外,还介绍了对合成酸对线粒体的影响以及对Jurkat肿瘤细胞可能的DNA损伤研究的结果。 -
Silver-Promoted Direct Phosphorylation of Bulky C(sp<sup>2</sup>)–H Bond to Build Fully Substituted β-Phosphonodehydroamino Acids作者:Hao-Qiang Cao、Hao-Nan Liu、Zhe-Yuan Liu、Baokun Qiao、Fa-Guang Zhang、Jun-An MaDOI:10.1021/acs.orglett.0c02229日期:2020.8.21A general and practical cross-dehydrogenative coupling protocol between readily available trisubstituted α,β-dehydro α-amino carboxylic esters and H-phosphites is described. This C(sp2)–H phosphorylation reaction proceeds with absolute Z-selectivity promoted by silver salt in a radical relay manner. The bulky tetrasubstituted β-phosphonodehydroamino acids were obtained in grams and added new modules
-
[EN] 3-MODIFIED ISO-/ISOALLO-LITHOCHOLIC ACID DERIVATIVES OR THEIR HOMO-ANALOGS FOR PREVENTING AND TREATING CLOSTRIDIOIDES DIFFICILE-ASSOCIATED DISEASES<br/>[FR] DÉRIVÉS D'ACIDE ISO-/ISOALLO-LITHOCHOLIQUE 3-MODIFIÉS OU LEURS HOMO-ANALOGUES POUR LA PRÉVENTION ET LE TRAITEMENT DE MALADIES ASSOCIÉES À CLOSTRIDIOIDES DIFFICILE申请人:PHENEX PHARMACEUTICALS AG公开号:WO2020260558A1公开(公告)日:2020-12-30The present invention relates to isolithocholic acid (3β-hydroxy-5β-cholan-24-oic acid) and isoallolithocholic acid (3β-hydroxy-5α-cholan-24-oic acid) together with the respective 22-homo-analogs or the deuterated analogs, which are modified in 3-position, for preventing or treating Clostridioides difficile-associated disease in a mammalian subject.
-
Lithocholic acid analogues, new and potent α-2,3-sialyltransferase inhibitors作者:Kai-Hsuan Chang、Lenselot Lee、Jessica Chen、Wen-Shan LiDOI:10.1039/b514915k日期:——A new type of noncompetitive α-2,3-sialyltransferase inhibitor has been synthesized; we report the discovery, preparation and inhibitory activity of sixteen lithocholic acid analogues.
-
Catalytic Oxidations of Steroid Substrates by Artificial Cytochrome P-450 Enzymes作者:Jerry Yang、Bartolo Gabriele、Sandro Belvedere、Ying Huang、Ronald BreslowDOI:10.1021/jo020174u日期:2002.7.1perform hydroxylations with substrate selectivity and regio- and stereoselectivity and high catalytic turnovers. The geometries of the catalyst/substrate complexes override intrinsic substrate reactivities, permitting attack on geometrically accessible saturated carbons of steroids in the presence of secondary carbinol groups and carbon-carbon double bonds, as in enzymatic reactions. Selective hydroxylations
表征谱图
-
氢谱1HNMR
-
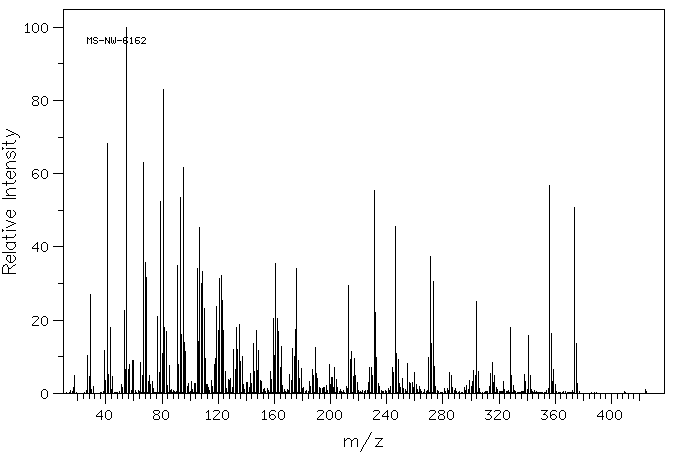
质谱MS
-
碳谱13CNMR
-
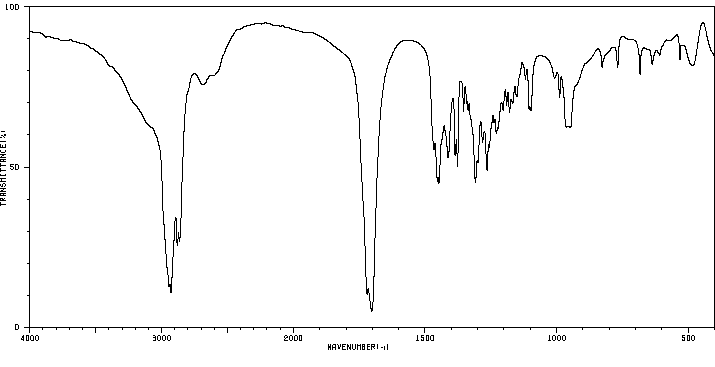
红外IR
-
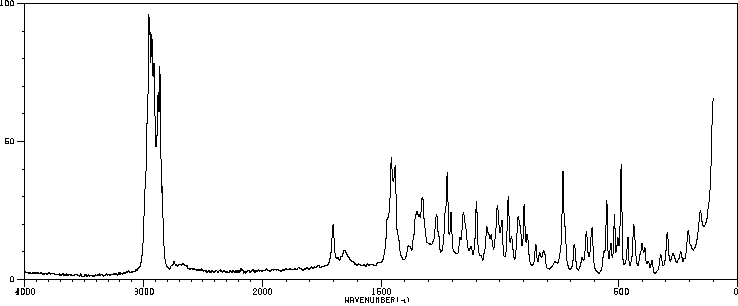
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息