异丁烷 | 75-28-5
物质功能分类
中文名称
异丁烷
中文别名
2-甲基丙烷;R-600a;R600A
英文名称
Isobutane
英文别名
HC600a;i-butane;2-methylpropane
CAS
75-28-5
化学式
C4H10
mdl
MFCD00008926
分子量
58.1234
InChiKey
NNPPMTNAJDCUHE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:−160 °C(lit.)
-
沸点:−12 °C(lit.)
-
密度:2.064 g/mL at 25 °C(lit.)
-
蒸气密度:2.01 (21 °C, vs air)
-
闪点:-83 °C
-
暴露限值:NIOSH REL: TWA 800 ppm (1,900 mg/m3).
-
LogP:2.760
-
物理描述:Colorless gas with a gasoline-like or natural gas odor. [Note: Shipped as a liquefied compressed gas. A liquid below 11°F.]
-
颜色/状态:Colorless gas [Note: Shipped as a liquified compressed gas. A liquid below 11 °F]
-
气味:Gasoline-like or natural gas odor.
-
溶解度:In water, 48.9 mg/L at 25 °C
-
蒸汽密度:2.01 (Air = 1)
-
蒸汽压力:2610 mm Hg (348.1 kPa) at 25 °C; 391.02 mm Hg (52.132 kPa) at -27.57 °C
-
大气OH速率常数:2.34e-12 cm3/molecule*sec
-
稳定性/保质期:
-
稳定性:稳定。
-
禁配物:强氧化剂、强酸、强碱、卤素。
-
聚合危害:不会发生聚合。
-
-
自燃温度:860 °F (460 °C) (Closed cup)
-
分解:When heated to decomposition it emits acrid smoke and irritating fumes.
-
粘度:0.238 cP at -10 °C
-
腐蚀性:No corrosive action on metals.
-
燃烧热:-680.84 kcal/mol at 25 °C (liquid); -685.71 kcal/mol at 25 °C (gas)
-
汽化热:4.570 kcal/mol at 25 °C
-
表面张力:14.1 dyne/cm at -10 °C
-
电离电位:10.74 eV
-
折光率:Index of refraction: 1.3518 at 25 °C/D
-
保留指数:354.2;353.99;353.53;353.53;353;354;366;361.1;362;366;365;365;369;359;354;354;366;354.8;362;366;354.2;372.8;368;354;364;354.2;354;354;366;366;355;354;370;366;370;368;364
计算性质
-
辛醇/水分配系数(LogP):2.1
-
重原子数:4
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
ADMET
代谢
进行了一项研究,以确定挥发性烃类化合物,如丙烷、正丁烷和异丁烷,是否在小鼠体内被代谢。在吸入这些气体的小鼠中,从丙烷中产生了异丙醇和丙酮,从正丁烷中产生了sec-丁醇和甲基乙基甲酮,从异丁烷中产生了tert-丁醇作为相应的代谢物。此外,发现肝微粒体含有参与这些代谢的酶系统。体外反应表明,肝微粒体可以将丙烷转化为异丙醇,将正丁烷转化为sec-丁醇,将异丁烷转化为tert-丁醇。据推测,烃类化合物首先被微粒体酶系统转化为(omega-1)-醇,然后通过醇脱氢酶转化为相应的酮。
A study was conducted to establish whether volatile hydrocarbons, such as propane, n-butane and iso-butane, are metabolized in mice or not. In mice having inhaled these gases, isopropanol and acetone were yielded from propane, sec-butanol and methyl ethyl ketone from n-butane, and tert-butanol from iso-butane as the respective metabolites. In addition, liver microsomes were found to contain the enzymic system participating in these metabolisms. In vitro reactions with liver microsomes produced isopropanol from propane, sec-butanol from n-butane, and tert-butanol from iso-butane. It was assumed that hydrocarbons were first converted to (omega-1)-alcohols by microsomal enzyme system and then to corresponding ketones by alcohol dehydrogenase.
来源:Hazardous Substances Data Bank (HSDB)
代谢
异丁烷在大鼠肝脏微粒体中被氧化代谢为其母醇。
Isobutane is oxidatively metabolized by rat liver microsomes to its parent alcohol.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
识别和使用:异丁烷是一种无色气体。它用于有机合成,作为制冷剂,在发动机燃料中,以及作为气溶胶推进剂,还用于合成橡胶和仪器校准液体中。人体研究:异丁烷是一种简单的窒息剂。急性暴露可能导致呼吸急促和心动过速。在严重情况下,可能出现低血压、呼吸暂停和心脏骤停。直接接触液体会产生化学烧伤。毒理学上,蒸汽对皮肤和眼睛没有影响。曾报告过一例因无意吸入空气清新剂中的异丁烷毒性导致的室颤。有意吸入挥发性物质(“嗅吸”)以获得兴奋和幻觉是一种儿童和青少年的物质滥用形式,其发病率和死亡率都很高。心脏心律失常、窒息或创伤可能导致突然死亡。曾报告过因嗅吸含有异丁烷的打火机填充物而导致死亡的案例。动物研究:将含有22%异丁烷的未稀释定型喷雾通过眼睛暴露给兔子的研究表明,眼睛刺激立即显现,出现短暂的虹膜炎和轻度结膜炎。狗急性暴露于55 mg/L异丁烷是致命的,而45 mg/L导致麻醉。将小鼠暴露于41 mg/L异丁烷两小时导致60%的暴露动物死亡,而暴露于52 mg/L的异丁烷在平均28分钟内对100%的动物致命。异丁烷在小鼠体内15%的浓度下60分钟和23%的浓度下26分钟时是一种中枢神经系统抑制剂。异丁烷在麻醉大鼠中引起呼吸暂停,最终导致心脏骤停。异丁烷在 Ames Salmonella 致突变性试验中测试结果为阴性。
IDENTIFICATION AND USE: Isobutane is a colorless gas. It is used in organic synthesis, as a refrigerant, in motor fuels, and as aerosol propellant, as well as in synthetic rubber, and in instrument calibration fluid. HUMAN STUDIES: Isobutane is a simple asphyxiant. Acute exposure may cause tachypnea and tachycardia. In severe cases, hypotension, apnea, and cardiac arrest develop. Direct contact with the liquid produces chemical burns. Toxicologically, the vapor exerts no effect on skin and eyes. A case of ventricular fibrillation due to isobutane toxicity after unintentional inhalation of air freshener has been reported. The intentional inhalation of a volatile substance ("sniffing") causing euphoria and hallucinations is a form of substance abuse in children and adolescents with a high morbidity and mortality. Sudden death can be caused by cardiac arrhythmia, asphyxia or trauma. Fatal cases of isobutane sniffing of cigarette lighter refill containing isobutane has been reported. ANIMAL STUDIES: Studies in rabbits exposed through the eyes to undiluted hairspray containing 22% isobutane showed that irritation of the eye was immediately evident with transient iritis and mild conjunctivitis. Acute exposure in dogs to 55 mg/L isobutane was fatal, and 45 mg/L caused anesthesia. Two-hour exposures of mice to 41 mg/L isobutane caused death in 60% of the exposed animals, whereas exposure to 52 mg/L was lethal to 100% of the animals within an average of 28 min. Isobutane is a CNS depressant in the mouse at 15% in 60 min, and at 23% in 26 min. Isobutane caused apnea and finally cardiac arrest in anesthetized rats. Isobutane tested negative in the Ames Salmonella mutagenicity assay.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
Butane is a simple asphyxiant and causes toxicity by displacing oxygen. It also affects the central nervous system by enhancing glycine receptors and inhibiting nicotinic acetylcholine and N-methyl-d-aspartate (NMDA) receptors, resulting in anesthetic effects. (L1284, A352)
来源:Toxin and Toxin Target Database (T3DB)
毒理性
对人类不具有致癌性(未被国际癌症研究机构IARC列名)。
No indication of carcinogenicity to humans (not listed by IARC).
来源:Toxin and Toxin Target Database (T3DB)
毒理性
Butane targets the central nervous system and cardiovascular system. Inhalation of butane can cause frostbite which can result in death from asphyxiation and ventricular fibrillation. (L1283, L1284)
来源:Toxin and Toxin Target Database (T3DB)
毒理性
该物质可以通过吸入被身体吸收。
The substance can be absorbed into the body by inhalation.
来源:ILO-WHO International Chemical Safety Cards (ICSCs)
安全信息
-
职业暴露等级:A
-
职业暴露限值:TWA: 800 ppm (1900 mg/m3)
-
危险等级:2.1
-
危险品标志:F+
-
安全说明:S,S16,S9
-
危险类别码:R12
-
WGK Germany:-
-
RTECS号:TZ4300000
-
海关编码:2901100000
-
危险类别:2.1
-
危险标志:GHS02,GHS04
-
危险品运输编号:UN 1969 2.1
-
危险性描述:H220,H280
-
危险性防范说明:P210,P377,P403,P410
-
储存条件:储存注意事项:储存于阴凉、通风的专用库房中,远离火种、热源,库温不宜超过30℃。应与氧化剂分开存放,切忌混储。使用防爆型照明和通风设施,并禁止使用易产生火花的机械设备和工具。储区应配备泄漏应急处理设备。
制备方法与用途
异丁烷简介
异丁烷是一种无色可燃性气体,微溶于水且性质稳定。其比重为0.5510(25℃),熔点-145℃,沸点-11.73℃。它存在于石油气、天然气及裂化气中,也可通过正丁烷异构化制得。异丁烷与空气可形成爆炸性混合物,爆炸极限为1.9%~8.4%(体积)。
化工应用异丁烷的化工利用主要包括四条途径:
国内主要用于直接用作民用燃料及烷基化生产车用燃料油调和成分,其他化工利用途径较少。异丁烷与丙烯共氧化法的优势在于无环境污染,但投资较高;相比之下,氯醇法虽污染严重,但在国内环氧丙烷的生产中普遍采用,其投资相对较低。
化学性质异丁烷是一种无色无臭易燃易爆气体,沸点约为-11℃。21℃时蒸气压约0.39MPa。
用途主要用途包括与异丁烯经烃化生产异辛烷,作为汽油辛烷值的改进剂;冷冻剂;石化企业分析、检测仪器的标准气体等。
生产方法天然存在于石油气、天然气和裂化气中。由碳四馏分分离或通过表面活性剂吸附及冷冻法从天然气中获得。
性质与安全 化学性质- 无色无臭易燃易爆气体,沸点约-11℃。
- 蒸汽压21℃时约0.39MPa。
主要用作石化企业分析、检测仪器的标准气。
生产方法天然存在于石油气、天然气和裂化气中。由碳四馏分分离或通过表面活性剂吸附及冷冻法从天然气获得。
安全性分类- 类别:有害气体。
- 毒性分级:低毒。
- 急性毒性(吸入):
- 大鼠LC50: 57,000ppm/15分;
- 小鼠LCL0: 104,100毫克/立方米/2小时。
与空气混合遇明火或受热可爆炸。
可燃性危险特性遇明火或受热可燃烧,产生刺激烟雾。
储运特性库房通风低温干燥;轻装轻卸;与氧气、空气等助燃气体钢瓶分开存放。
灭火剂 职业标准- 时间加权平均容许浓度(TWA):1430毫克/立方米。
- 短时间接触容许浓度(STEL):1800毫克/立方米。
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:Catalytic synthesis of unsaturated nitriles from NO–alkane or NO–alkene on Pt–Sn/SiO2摘要:通过选择性双金属CVD反应制备的Pt–Sn/SiO2催化剂,以SnMe4与SiO2上的Pt颗粒反应,并通过EXAFS(扩展X射线吸收精细结构)表征,能够从NO + 丙烯产生丙烯腈,从NO + 异丁烯和NO + 异丁烷产生甲基丙烯腈,选择性良好,通常为70–93%,相比之下,单金属Pt/SiO2催化剂的活性和选择性可以忽略不计(大约低一个数量级)。DOI:10.1039/c39950000329
-
作为产物:参考文献:名称:Group 4 metal halides/alumina solid superacids Relation between electronegativity, acid strength and catalytic properties摘要:通过四族金属卤化物蒸气对铝土矿的作用获得的固体超酸的催化性质与所用卤化物的电负性相关。研究发现,路易斯超酸中心的酸性强度和低温五烷异构化的反应路径取决于与铝土矿载体结合的金属卤化物物种的电子接受能力。DOI:10.1039/a707395j
-
作为试剂:参考文献:名称:Hydride ion transfer reactions in the gas phase. Pressure dependence of reaction efficiency as a criterion for the recognition of anchimeric assistance摘要:气相中氢化物离子从2,2-二甲基丁烷向叔丁基碳正离子的传递效率(kobs/kcoll)显示出明显的正压力依赖性,这表明过渡态中与离去CH2氢化物相邻的2,2-二甲基丁烷甲基基团具有显著的邻位促进作用。DOI:10.1039/c39950000121
文献信息
-
Gas-phase reactions of iron(1-) and cobalt(1-) with simple thiols, sulfides, and disulfides by Fourier-transform mass spectrometry作者:L. Sallans、K. R. Lane、B. S. FreiserDOI:10.1021/ja00185a013日期:1989.2products, H-Fesup minus}}-SH and Fesup minus}}-SH. Some of the thermochemical data derived from this study include Ddegree}(Msup minus}}-S) > 103 kcal/mol and Ddegree}(Msup minus}}-SH) = 83 plus minus}9 kcal/mol. Finally, a brief survey of the reactivity of Vsup minus}}, Crsup minus}}, and Mosup minus}} with selected organosulfur compounds is also reported. 79 refs., 3 figs., 7 tabs发现 Fesup minus}} 和 Cosup minus}} 会与简单的硫醇、硫化物和二硫化物反应。由这些金属阴离子 Msup minus}} 和硫醇形成的主要反应产物包括 MSsup minus}}、MSHsup minus}} 和 MSHsub 2}sup minus}} 和提出了一种涉及金属初始插入弱 CS 键的机制。类似地,CS 插入是与硫化物和二硫化物反应的主要攻击模式,类似于观察到的金属阳离子反应。碰撞诱导解离用于支持主要产物 H-Fesup minus}}-SH 和 Fesup minus}}-SH 的拟议结构。本研究得出的一些热化学数据包括 Ddegree}(Msup minus}}-S) > 103 kcal/mol 和 Ddegree}(Msup minus}}-SH) = 83 plus减去}9 kcal/mol。最后,还报告了
-
Synthesis of Al-MTW with low Si/Al ratios by combining organic and inorganic structure directing agents
-
Process for the preparation of optically active piperazine-2-carboxylic申请人:Lonza, Ltd.公开号:US05886181A1公开(公告)日:1999-03-23Optically active piperazine-2-carboxylic acid derivatives of the general formula: ##STR1## wherein R.sup.1 and R.sup.2 are inter alia hydrogen, alkyl or acyl and X is alkoxy or a (substituted) amino group, are prepared by asymmetric hydrogenation of the corresponding 1,4,5,6-tetrahydropyrazines, catalyzed by optically active rhodium, ruthenium or iridium complexes. The compounds of the Formula 1 are intermediates for the preparation of pharmaceutical active ingredients, for example, HIV protease inhibitors.
-
Palladium(0)-Catalyzed Cross-Coupling of 1,1-Diboronates with Vinyl Bromides and 1,1-Dibromoalkenes作者:Huan Li、Zhikun Zhang、Xianghang Shangguan、Shan Huang、Jun Chen、Yan Zhang、Jianbo WangDOI:10.1002/anie.201407000日期:2014.10.27Palladium‐catalyzed cross‐coupling reactions of 1,1‐diboronates with vinyl bromides and dibromoalkenes were found to afford 1,4‐dienes and allenes, respectively. These reactions utilize the high reactivities of both 1,1‐diboronates and allylboron intermediates generated in the initial coupling.
-
Dehydrogenation and Isomerization of Butane over Cr Catalysts Supported on H-SSZ-35 Type Zeolites作者:Megumu Inaba、Kazuhisa Murata、Masahiro Saito、Isao Takahara、Naoki Mimura、Hideaki Hamada、Yohei KurataDOI:10.1246/bcsj.77.381日期:2004.2The dehydrogenation and isomerization of butane to isobutene over Cr catalysts supported on zeolites were investigated. Although a zeolite support, especially with a low Si/Al 2 ratio, favors the cracking of butane over its dehydrogenation and isomerization, due to strong solid acidity, the loading of Cr on a zeolite support enhances the dehydrogenation and isomerization of butane. H-SSZ-35 type zeolite研究了在沸石负载的铬催化剂上丁烷脱氢和异构化成异丁烯。尽管沸石载体,尤其是具有低Si/Al 2 比的沸石载体有利于丁烷的裂解而不是其脱氢和异构化,但由于强固体酸性,沸石载体上的Cr负载增强了丁烷的脱氢和异构化。H-SSZ-35 型沸石具有一维笼型通道结构,采用顺式、顺式、顺式-N-甲基六氢十里鎓氢氧化物作为结构方向凝胶(SDA)水热合成我们发现 Cr/H-SSZ-35 (Si/Al 2 = 500) 显示出中等的丁烷转化率和异丁烯选择性。因此,在本研究中使用的催化剂中,异丁烯的产率(丁烷转化率 x 异丁烯的选择性)是最高的。它在 500°C 下的活性在 6 小时内保持不变。我们的证据表明,丁烷在沸石载体或 Cr 2 O 3 颗粒上脱氢,一部分脱氢产物在沸石载体上异构化为异丁烯。
表征谱图
-
氢谱1HNMR
-
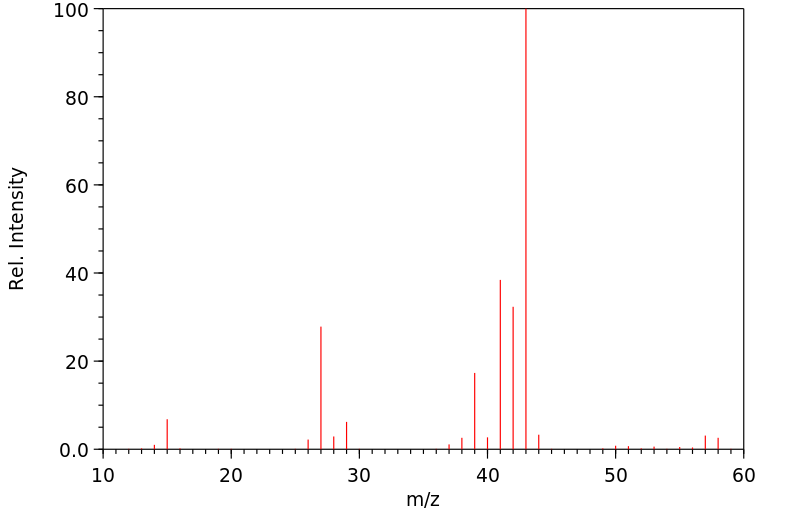
质谱MS
-
碳谱13CNMR
-
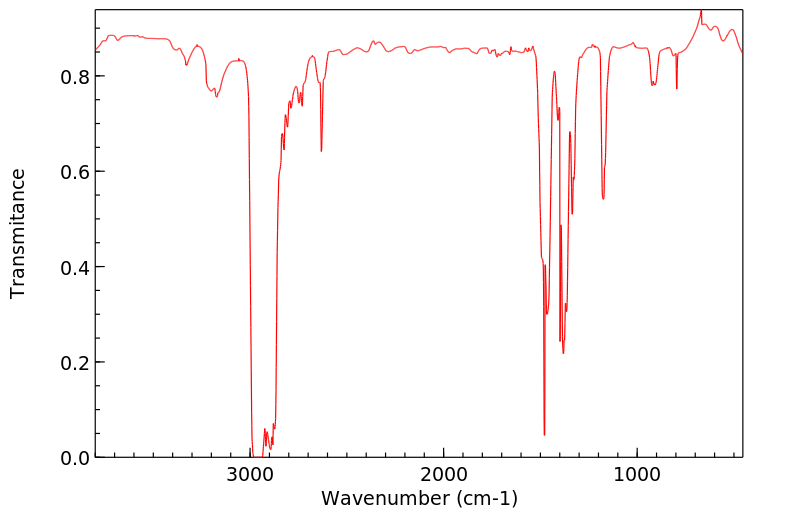
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式-1-乙基-3-甲基环己烷
顺式-1-乙基-2-甲基环丙烷
顺式-1,3-二甲基环庚烷
顺式-1,2-二甲基环丙烷
顺式-1,2-二乙基环戊烷
顺式-1,2-二(1-甲基乙基)环丙烷
顺式-1,2-二(1-甲基乙基)环丙烷
顺式,反式,反式-1,2,4-三甲基环己烷
Copper, ethyl-
辛烷-d18
辛基环戊烷
辛基环丙烷
联苯肼酯
联环戊基
羰基双(环茂二烯基)钛
矿油精
癸烷,2,8-二甲基-
癸烷
decyl radical
癸基环戊烷
異十八烷
甲烷-d3
甲烷-d2
甲烷-d1
甲烷-D4
甲烷-3H
甲烷-13C,d4
甲烷-13C
甲烷
甲基自由基
甲基环辛烷
甲基环癸烷
甲基环戊烷
甲基环己烷-Me-d3
甲基环己烷
甲基环十一烷
甲基环丙烷
甲基环丁烷.
甲基丙烷-2-d
环辛烷-D16
环辛烷
环癸烷
环戊烷-D9
环戊烷-D10
环戊烷-13C1
环戊烷,三(2-辛基十二基)-
环戊烷
环戊基甲基自由基
环戊基环庚烷
环戊基环己烷