1,1,1,5,5,6,6,6-八氟-2,4-己二酮 | 20825-07-4
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:85-86 °C (lit.)
-
密度:1.538 g/mL at 25 °C (lit.)
-
闪点:>230 °F
-
稳定性/保质期:
避免接触强氧化物和强还原剂。
计算性质
-
辛醇/水分配系数(LogP):2.7
-
重原子数:16
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.666
-
拓扑面积:34.1
-
氢给体数:0
-
氢受体数:10
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xn,Xi
-
危险类别码:R20/21/22
-
危险品运输编号:UN 3265
-
海关编码:2914700090
-
安全说明:S26,S36
-
WGK Germany:3
-
储存条件:将物品存放在阴凉、干燥的密闭容器中保存。
SDS
| Name: | 1 1 1 5 5 6 6 6-Octafluoro-2 4-Hexanedione Tech. 90% Material Safety Data Sheet |
| Synonym: | None |
| CAS: | 20825-07-4 |
Synonym:None
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 20825-07-4 | 1,1,15,5,6,6,6-Octafluoro-2,4-Hexanedi | 90% | unlisted |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
Causes eye irritation.
Skin:
Causes skin irritation.
Ingestion:
Causes gastrointestinal irritation with nausea, vomiting and diarrhea. The toxicological properties of this substance have not been fully investigated.
Inhalation:
Causes respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Use water spray to keep fire-exposed containers cool. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas. Containers may explode when heated.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Cool containers with flooding quantities of water until well after fire is out. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Avoid runoff into storm sewers and ditches which lead to waterways. Clean up spills immediately, observing precautions in the Protective Equipment section. Remove all sources of ignition. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use with adequate ventilation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed.
Avoid ingestion and inhalation. Wash clothing before reuse.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 20825-07-4: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: colorless
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 85 - 86 deg C
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: > 110 deg C (> 230.00 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: 1.5380g/cm3
Molecular Formula: C6H2F8O2
Molecular Weight: 258.07
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Incompatible materials, ignition sources, excess heat.
Incompatibilities with Other Materials:
Strong acids, strong bases, strong oxidizing agents, strong reducing agents.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide, hydrogen fluoride gas.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 20825-07-4 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
1,1,15,5,6,6,6-Octafluoro-2,4-Hexanedione - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 37/39 Wear suitable gloves and eye/face
protection.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 20825-07-4: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 20825-07-4 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 20825-07-4 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:描述:1,1,1,5,5,6,6,6-八氟-2,4-己二酮 在 一水合肼 、 四磷十氧化物 作用下, 以 氯仿 为溶剂, 反应 4.0h, 以87%的产率得到5-(pentafluoroethyl)-3-(trifluoromethyl)-1H-pyrazole参考文献:名称:三氟甲基取代的吡唑和相应的β-二酮的新合成途径摘要:一种改进的,更有效的三氟甲基取代的吡唑合成方法,即3,5-双(三氟甲基)-1 H-吡唑(1a),5-(五氟乙基)-3-(三氟甲基)-1 H-吡唑(1b), 5-(七氟丙基)-3-(三氟甲基)-1 H-吡唑(1c),5-(九氟丁基)-3-(三氟甲基)-1 H-吡唑(1d),5-苯基-3-(三氟甲基)- 1 H-吡唑(1g),5-(五氟苯基)-3-(三氟甲基)-1 H-吡唑(1h),5-甲基-3-(三氟甲基)-1 H-吡唑(1e)和5-(叔丁基)-3-(三氟甲基)-1 H-吡唑(1f)以相应的β-二酮(2a – h)为起点。此外,重新讨论了一些相应的二酮(2b - d和2h)的制备,重点是低成本和易于获得的原料。所有产品均使用多核(1 H,13 C,19 F)NMR光谱进行表征。另外,使用差示扫描量热法表征了合成的吡唑的热性质。DOI:10.1016/j.tetlet.2016.02.092
-
作为产物:描述:bis(1,1,1,5,5,6,6,6-octafluorohexane-2,4-dionato)copper(II) 在 硫酸 作用下, 以11.6 g的产率得到1,1,1,5,5,6,6,6-八氟-2,4-己二酮参考文献:名称:三氟甲基取代的吡唑和相应的β-二酮的新合成途径摘要:一种改进的,更有效的三氟甲基取代的吡唑合成方法,即3,5-双(三氟甲基)-1 H-吡唑(1a),5-(五氟乙基)-3-(三氟甲基)-1 H-吡唑(1b), 5-(七氟丙基)-3-(三氟甲基)-1 H-吡唑(1c),5-(九氟丁基)-3-(三氟甲基)-1 H-吡唑(1d),5-苯基-3-(三氟甲基)- 1 H-吡唑(1g),5-(五氟苯基)-3-(三氟甲基)-1 H-吡唑(1h),5-甲基-3-(三氟甲基)-1 H-吡唑(1e)和5-(叔丁基)-3-(三氟甲基)-1 H-吡唑(1f)以相应的β-二酮(2a – h)为起点。此外,重新讨论了一些相应的二酮(2b - d和2h)的制备,重点是低成本和易于获得的原料。所有产品均使用多核(1 H,13 C,19 F)NMR光谱进行表征。另外,使用差示扫描量热法表征了合成的吡唑的热性质。DOI:10.1016/j.tetlet.2016.02.092
文献信息
-
EUROPIUM COMPLEX申请人:TOSOH CORPORATION公开号:US20200354389A1公开(公告)日:2020-11-12To provide europium complexes having high photostability. A europium complex expressed with the following formula (A): wherein, R A and R B are independently a cyclic alkyl group with 3 to 10 carbons, respectively, and R C is a cyclic alkyl group with 3 to 10 carbons or a phenyl group expressed with the following formula (B): (wherein, X A , X B , A C , X D and X E independently represent a hydrogen atom; a fluorine atom; an alkyl group with 1 to 3 carbon(s); an alkyloxy group with 1 to 3 carbon(s); an aryloxy group with 6 to 10 carbons; a fluoroalkyl group with 1 to 3 carbon(s); a fluoroalkyloxy group with 1 to 3 carbon(s); or a phenyl group that may be substituted with a fluorine atom, an alkyl group with 1 to 3 carbon(s), an alkyloxy group with 1 to 3 carbon(s), a fluoroalkyl group with 1 to 3 carbon(s), a fluoroalkyloxy group with 1 to 3 carbon(s), a fluorophenyl group, a hydroxyl group or a cyano group, respectively); R A is a cyclic alkyl group with 3 to 10 carbons; R B and R C are a phenyl group expressed with the formula (B), provided, however, that a case where R A a cyclohexyl group, and, R B and R C are a phenyl group is excluded; or R A , R B and R C independently represent an ortho-substituted phenyl group expressed with the following formula (Ba): (wherein, X E represents a hydrogen atom, an alkyl group with 1 to 3 carbon(s), an alkyloxy group with 1 to 3 carbon(s), a fluoroalkyl group with 1 to 3 carbon(s), a fluoroalkyloxy group with 1 to 3 carbon(s), a naphthyl group that may be substituted with a fluorine atom, a pyridyl group that may be substituted with a fluorine atom, or a phenyl group that is expressed with a formula (C): [wherein, Z A , Z C and Z E independently represent a hydrogen atom, a fluorine atom, an alkyl group with 1 to 3 carbon(s), an alkyloxy group with 1 to 3 carbon(s), a fluoroalkyl group with 1 to 3 carbon(s), a fluoroalkyloxy group with 1 to 3 carbon(s), a phenyl group that may be substituted with a fluorine atom, a hydroxyl group or a cyano group; Z B and Z D independently represent a hydrogen atom or a fluorine atom, respectively], provided, however, that a case where R A , R B and R C are all a phenyl group is excluded), respectively; R D represents a hydrogen atom, a deuterium atom or a fluorine atom; W A and W B independently represent an alkyl group with 1 to 6 carbon(s), a fluoroalkyl group with 1 to 6 carbon(s), a phenyl group, a 2-thienyl group or a 3-thienyl group; and ‘n’ represents an integer of 1 to 3}.提供具有高光稳定性的铕配合物。 以下是用以下公式(A)表示的铕配合物: 其中,R A 和R B 分别独立地是具有3至10个碳原子的环烷基基团,而R C 是具有3至10个碳原子的环烷基基团或用以下公式(B)表示的苯基团: (其中,X A 、X B 、A C 、X D 和X E 独立地代表氢原子;氟原子;具有1至3个碳原子的烷基基团;具有1至3个碳原子的烷氧基团;具有6至10个碳原子的芳基氧基团;具有1至3个碳原子的氟烷基基团;具有1至3个碳原子的氟烷氧基团;或者可能被氟原子、具有1至3个碳原子的烷基基团、具有1至3个碳原子的烷氧基团、具有1至3个碳原子的氟烷基基团、具有1至3个碳原子的氟烷氧基团、氟苯基团、羟基或氰基取代的苯基团,分别); R A 是具有3至10个碳原子的环烷基基团; R B 和R C 是用公式(B)表示的苯基团,但是,排除R A 为环己基团,且R B 和R C 为苯基团的情况;或者 R A ,R B 和R C 独立地表示用以下公式(Ba)表示的邻位取代的苯基团: (其中,X E 代表氢原子、具有1至3个碳原子的烷基基团、具有1至3个碳原子的烷氧基团、具有1至3个碳原子的氟烷基基团、具有1至3个碳原子的氟烷氧基团、可能被氟原子取代的萘基团、可能被氟原子取代的吡啶基团,或者用以下公式(C)表示的苯基团: [其中,Z A 、Z C 和Z E 独立地代表氢原子、氟原子、具有1至3个碳原子的烷基基团、具有1至3个碳原子的烷氧基团、具有1至3个碳原子的氟烷基基团、具有1至3个碳原子的氟烷氧基团、可能被氟原子取代的苯基团、羟基或氰基;Z B 和Z D 独立地代表氢原子或氟原子,但排除R A ,R B 和R C 都是苯基团的情况),分别;R D 代表氢原子、氘原子或氟原子;W A 和W B 独立地表示具有1至6个碳原子的烷基基团、具有1至6个碳原子的氟烷基基团、苯基、2-噻吩基团或3-噻吩基团;‘n’表示1至3的整数。
-
[EN] N1 - ((PYRAZOL-1-YMETHYL) -2-METHYLPHENYL)- PHATALAMIDE DERIVATIVES AND RELATED COMPOUNDS INSECTICIDES<br/>[FR] DERIVES DE N1 - ((PYRAZOL-1-YMETHYL) -2-METHYLPHENYL)- PHATALAMIDE ET COMPOSES INSECTICIDES ASSOCIES申请人:BAYER CROPSCIENCE AG公开号:WO2005095351A1公开(公告)日:2005-10-13Novel benzenedicarboxamides of the formula (I) wherein X represents hydrogen, halogen atom, nitro, C1-6alkylsulfonyloxy, C1-6alkylsulfinyl, C1-6alkylsulfenyl or C1-6alkylsulfonyl, R1 represents C1-6alkyl, C1-6alkylthio-C1-6alkyl, C1-6alkylsulfinyl- C1-6alkyl or C1-6 alkylsulfonyl- C1-6alkyl, Y represents halogen or C1-6alkyl, m represents 0 or 1, A represents O, S, SO, SO2, CH2 or CH(CH3), and Q represents a 5- or 6-membered heterocyclic group that contains at least one hetero atom selected from the group consisting of N, O and S and can be optionally substituted; processes for their preparation, their intermediates and their use as insecticides.
-
<i>In vitro</i> and <i>In Vivo</i> Evaluation of Quinoxaline 1,4-di-N-oxide Against <i>Giardia lamblia</i>作者:Elizabeth Barbosa-Cabrera、Rosa Moo-Puc、Antonio Monge、Alma Delia Paz-González、Virgilio Bocanegra-García、Gildardo RiveraDOI:10.2174/1570180816666190618115854日期:2020.4.25
Background: Giardiasis is an important public health problem. However, its pharmacological treatment is limited mainly to two drugs, metronidazole and nitazoxanide. Objectives: Screening four series of esters (methyl, ethyl, isopropyl and n-propyl) of quinoxaline-7- carboxylate 1,4-di-N-oxide in in vitro and in vivo models as antigiardiasis agents.
Objectives: Screening four series of esters (methyl, ethyl, isopropyl and n-propyl) of quinoxaline-7- carboxylate 1,4-di-N-oxide in in vitro and in vivo models as antigiardiasis agents.
Methods: Briefly, 4 × 104 trophozoites of G. lamblia were incubated for 48 h at 37 °C with different concentrations of esters of quinoxaline-7-carboxylate 1,4-di-N-oxide, albendazole, metronidazole and nitazoxanide. Afterwards, trophozoites were counted and the half maximal inhibitory concentration (IC50) was calculated by Probit analysis. The in vivo antigiardial activity of the compounds was demonstrated using experimental infections of G. lamblia in suckling female CD-1 mice.
Results: Compound T-069 with a thienyl, a trifluoromethyl and an isopropyl group at R1-, R2- and R3-position, respectively, on the quinoxaline 1,4-di-N-oxide ring in an in vitro model showed an IC50 value of 0.0014 µM, and 3502 and 1108 times more giardicidal activity than nitazoxanide and metronidazole in an in vivo model.
Conclusion: Isopropyl ester of quinoxaline-7-carboxylate 1,4-di-N-oxide derivatives showed better giardicidal activity than the reference drugs; therefore, these compounds are good candidates to develop new pharmacological treatment for giardiasis.
背景:贾第虫病是一个重要的公共卫生问题。然而,其药物治疗主要局限于两种药物,甲硝唑和硝唑酮。目标:在体外和体内模型中筛选四系列喹诺酮-7-羧酸二-N-氧化物(甲基、乙基、异丙基和正丙基)的酯类化合物作为抗贾第虫药剂。 目标:在体外和体内模型中筛选四系列喹诺酮-7-羧酸二-N-氧化物(甲基、乙基、异丙基和正丙基)的酯类化合物作为抗贾第虫药剂。 方法:简而言之,将4 × 104个贾第虫滋养体与不同浓度的喹诺酮-7-羧酸二-N-氧化物酯类、阿苯达唑、甲硝唑和硝唑酮一起在37°C下孵育48小时。然后,对滋养体进行计数,并通过Probit分析计算半最大抑制浓度(IC50)。这些化合物的体内抗贾第虫活性是通过在哺乳期雌性CD-1小鼠中进行贾第虫实验性感染来证明的。 结果:在体外模型中,具有噻吩基、三氟甲基和异丙基基团分别位于喹诺酮-7-羧酸二-N-氧化物环的R1、R2和R3位置的化合物T-069显示出IC50值为0.0014微米,在体内模型中的贾第杀灭活性比硝唑酮和甲硝唑分别高出3502倍和1108倍。 结论:喹诺酮-7-羧酸二-N-氧化物的异丙基酯衍生物显示出比参考药物更好的贾第杀灭活性;因此,这些化合物是开发新的贾第虫病药物治疗的良好候选。 -
Synthesis, NMR, photoluminescence studies and intramolecular energy transfer process of europium(III) complexes作者:Manju Bala、Satish Kumar、Rekha Devi、V.B. Taxak、Priti Boora、S.P. KhatkarDOI:10.1016/j.jfluchem.2016.07.005日期:2016.8The studies related to the synthesis and luminescence properties of five europium(III) complexes of the type [Eu(OFHD)L] where OFHD is deprotonated 1,1,1,5,5,6,6,6-octafluorohexane-2,4-dione and L is H2O (C1), 2,2′-biquinoline (C2), neocuproine (C3), 1,10-phenanthroline (C4) and 2,2′- bipyridyl (C5) are reported in the present communication. The synthesized complexes were investigated by means of elemental有关五种类型[Eu(OFHD)L]的euro(III)配合物的合成和发光特性的研究,其中OFHD被1,1、1,1,5,5,6,6,6-八氟己烷-2去质子化,据报道,4-二酮和L为H 2 O(C1),2,2'-联喹啉(C2),新铜嘌呤(C3),1,10-菲咯啉(C4)和2,2'-联吡啶(C5)。目前的沟通。通过元素分析,IR,1 H NMR,紫外可见光谱和TGA技术研究了合成的配合物。配合物中的高氟化度使其成为NMR位移试剂的最佳候选物,以解析光谱,如图1所示。1 H NMR研究。详细研究了固态配合物的激发和发射光谱。此外,基于发射光谱,CIE颜色坐标(x和y),发光衰减时间(τ),辐射衰减率(A rad),非辐射率(A nrad),量子效率(η)和Judd-Ofelt强度参数(Ω 2)进行了计算和分析。提议的分子内能量转移机制代表了配体的最低三重态能级与the(III)离子的发射能级之间的
-
N1-((Pyrazol-1-Ymethyl)-2-Methylphenyl)-Phatalamide Derivatives And Related Compounds Insecticides申请人:Wada Katsuaki公开号:US20070299085A1公开(公告)日:2007-12-27Novel benzenedicarboxamides of the formula (I) wherein X represents hydrogen, halogen atom, nitro, C 1-6 alkylsulfonyloxy, C 1-6 alkylsulfinyl, C 1-6 alkylsulfenyl or C 1-6 alkylsulfonyl, R 1 represents C 1-6 alkyl, C 1-6 alkylthio-C 1-6 alkyl, or C 1-6 alkyl, m represents 0 or 1, A represents O, S, SO, SO 2 , CH 2 or CH(CH 3 ), and Q represents a 5- or 6-membered heterocyclic group that contains at least one hetero atom selected from the group consisting of N, O and S and can be optionally substituted; processes for their preparation, their intermediates and their use as insecticides.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
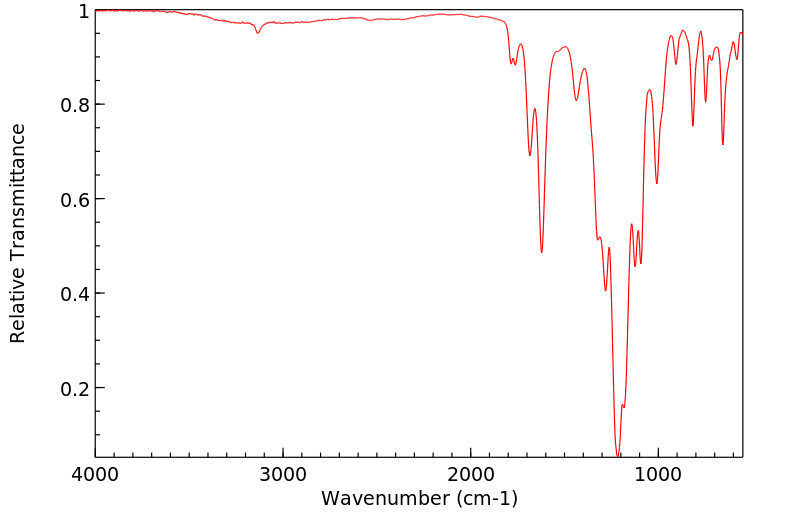
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息