1,2,3-三氯丙烷 | 96-18-4
中文名称
1,2,3-三氯丙烷
中文别名
三氯丙烷
英文名称
1,2,3-trichloropropane
英文别名
TCP
CAS
96-18-4
化学式
C3H5Cl3
mdl
MFCD00000946
分子量
147.432
InChiKey
CFXQEHVMCRXUSD-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-14 °C (lit.)
-
沸点:156 °C (lit.)
-
密度:1.387 g/mL at 25 °C (lit.)
-
闪点:180 °F
-
溶解度:Soluble in alcohol and ether (Weast, 1986); miscible with propyl chloride, carbon tetrachloride, and chloroform.
-
暴露限值:TLV-TWA 10 ppm (~60 mg/m3) (ACGIH), 50 ppm (MSHA, OSHA, and NIOSH); IDLH 1000 ppm (NIOSH).
-
介电常数:2.3(29℃)
-
LogP:2.63 at 25℃
-
物理描述:1,2,3-trichloropropane is a colorless liquid with a strong acid odor. Denser than water and slightly soluble in water. Hence sinks in water. (USCG, 1999)
-
颜色/状态:Colorless liquid
-
气味:Odor described as being quite similar to that of trichloroethylene or chloroform
-
蒸汽密度:5.08 (NTP, 1992) (Relative to Air)
-
蒸汽压力:3.69 mm Hg at 25 °C
-
亨利常数:3.43e-04 atm-m3/mole
-
稳定性/保质期:
-
自燃温度:304 °C
-
分解:When heated to decomposition it yields highly toxic /chloride./
-
粘度:2.5X10-4 Pa.s at 20 °C
-
燃烧热:-1.5536X10+9 J/kmol
-
汽化热:4.7998X10+7 J/kmole
-
表面张力:3.77X10-2 N/m at 20 °C
-
折光率:Index of refraction = 1.4852 at 20 °C/D
-
保留指数:878.4;883;895.5;889.3;890.6;890;899;899;910;894.2;910
计算性质
-
辛醇/水分配系数(LogP):1.8
-
重原子数:6
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
ADMET
代谢
F-344雄性大鼠通过腹腔注射给予30毫克/千克[(14)C]-1,2,3-三氯丙烷(TCP)(每千克100微居里),并在4小时后处死。与肝脏蛋白质、DNA和RNA共价结合的程度分别为418、244和432皮摩尔[(14)C]TCP当量/毫克。体内的共价结合时间过程显示,与肝脏DNA结合的[(14)C]TCP当量没有显著变化(1-48小时),而与蛋白质的结合在4小时时达到最大,并在48小时时显著减少。当两次和三次剂量间隔24小时给予时,TCP相关放射活性与肝脏蛋白质和DNA的结合被证明是累积的。用苯巴比妥预处理动物导致共价结合减少,而用SKF 525-A预处理导致共价结合增加。用β-萘黄酮预处理大鼠并没有改变[(14)C]TCP当量与蛋白质或DNA的共价结合。然而,用L-丁硫氨酸-(R,S)-亚砜胺耗尽谷胱甘肽(GSH)可使蛋白质结合增加342%,而DNA结合减少56%。腹腔注射TCP也在30和100毫克/千克剂量后的2小时内使肝脏GSH减少了41%和61%。体内的结合数据表明GSH在TCP的生物活化中具有双重作用。部分原因可能是GSH参与了TCP与肝脏DNA的生物活化和共价结合。然而,它也似乎能解毒与蛋白质结合的反应中间体。
... Male F-344 rats were administered 30 mg/kg [(14)C]- 1,2,3-trichloropropane (TCP) (100 uCi/kg) ip and killed 4 hr later. The extent of covalent binding to hepatic protein, DNA, and RNA was 418, 244, and 432 pmol [(14)C]TCP equivalents/mg, respectively. An in vivo covalent binding time course showed no significant change in [(14)C]TCP equivalents bound to hepatic DNA (1-48 hr), while binding to protein was maximal by 4 hr and decreased significantly by 48 hr. The binding of TCP-associated radioactivity to hepatic protein and DNA was shown to be cumulative for two and three doses when given 24 hr apart. Pretreatment of animals with phenobarbital caused a decrease while pretreatment with SKF 525-A caused an increase in covalent binding of [(14)C]TCP equivalents to protein and DNA. Pretreatment of rats with beta-naphthoflavone did not alter the covalent binding of [(14)C]TCP equivalents to protein or DNA. However, glutathione depletion with L-buthionine-(R,S)-sulfoximine increased binding to protein by 342% while it decreased binding to DNA by 56%. Intraperitoneal administration of TCP also depleted hepatic GSH by 41 and 61% 2 hr after doses of 30 and 100 mg/kg. The in vivo binding data suggest a dual role for GSH in the bioactivation of TCP. It may, in part, be that GSH is involved in the bioactivation and covalent binding of TCP to hepatic DNA. However, it also appears to detoxify a reactive intermediate(s) that binds to protein.
来源:Hazardous Substances Data Bank (HSDB)
代谢
在大鼠(30 mg/kg)和无胸腺小鼠(30和60 mg/kg)口服急性暴露于1,2,3-三氯丙烷(TCP)后,该物质被迅速吸收、代谢和排泄...通过核磁共振、质谱分析和与合成标准的比较,分离并鉴定出两种尿液代谢物,分别为N-乙酰基-和S-(3-氯-2-羟基丙基)半胱氨酸。对早期尿液(0-6小时)的分析表明,这种巯基尿酸在大鼠尿液中是主要的代谢物,而在小鼠尿液中只是一个小成分。通过核磁共振和质谱以及化学合成鉴定出的2-(S-谷胱甘肽)丙二酸是大鼠胆汁中的主要代谢物。
Following acute oral exposure of male and female F344 rats (30 mg/kg) and male B6C3F1 mice (30 and 60 mg/kg), 1,2,3-trichloropropane (TCP) was rapidly absorbed, metabolized, and excreted ... Two urinary metabolites were isolated and identified by NMR, mass spectroscopy, and comparison with synthetic standards, as N-acetyl- and S-(3-chloro-2-hydroxypropyl)cysteine. Analyses of the early urine (0-6 hr) showed this mercapturic acid to be the major metabolite in rat urine and was only a minor component in mouse urine. 2-(S-Glutathionyl)malonic acid was identified by NMR and mass spectrometry and by chemical synthesis as the major biliary metabolite in rats.
来源:Hazardous Substances Data Bank (HSDB)
代谢
在大鼠和人体肝脏微粒体的体外研究中,已经发现卤代烃1,2,3-三氯丙烷(TCP)被生物激活为直接作用的诱变剂1,3-二氯丙酮(DCA)。通过气相色谱-质谱联用技术确认了微粒体培养中DCA的存在。DCA的形成完全依赖于NADPH的存在。使用大鼠和人体微粒体形成DCA的速率分别为0.268 +/- 0.029和0.026 +/- 0.006 nmol/min/mg蛋白 +/- 标准误, respectively。当肝脏微粒体从用细胞色素P-450诱导剂,苯巴比妥和地塞米松预处理的大鼠中分离出来时,观察到DCA产生速率分别增加了24倍和2.5倍。用β-萘黄酮预处理导致DCA形成减少了50%。细胞色素P-450的抑制剂SKF 525-A和1-氨基苯并三唑分别产生了85%和70%的DCA形成抑制。当酒精脱氢酶和NADH被添加到微粒体培养中时,形成了两种与TCP相关的醇,1,3-二氯-2-丙醇和2,3-二氯丙醇。这些醇是初始微粒体代谢物DCA和2,3-二氯丙醛的产物。[14C]TCP等价物与大鼠肝脏微粒体蛋白共价结合。当使用苯巴比妥预处理的大鼠肝脏微粒体时,这种结合增加了8倍。向培养物中添加谷胱甘肽或N-乙酰半胱氨酸完全抑制了这种结合。在N-乙酰半胱氨酸存在下,1,3-(2-丙酮)-双-S-(N-乙酰半胱氨酸)(PDM)是唯一检测到的N-乙酰半胱氨酸结合物。它代表了TCP微粒体代谢的87%。PDM的形成表明DCA是TCP的主要微粒体蛋白结合代谢物。DCA的形成,作为一种直接作用的诱变剂,可能是大鼠肝脏微粒体系统中TCP诱变性的原因...
In vitro studies using rat and human hepatic microsomes have shown that the halogenated hydrocarbon 1,2,3-trichloropropane (TCP) is bioactivated to the direct acting mutagen 1,3-dichloroacetone (DCA). The presence of DCA in microsomal incubations was confirmed by gas chromatography-mass spectrometry. DCA formation was totally dependent on the presence of NADPH. The rate of DCA formation using rat and human microsomes was 0.268 +/- 0.029 and 0.026 +/- 0.006 nmol/min/mg protein +/- SE, respectively. When hepatic microsomes were isolated from rats pretreated with the cytochrome P-450 inducers, phenobarbital, and dexamethasone, 24- and 2.5-fold increases, respectively, in the rate of DCA production, were observed. Pretreatment with beta-naphthoflavone resulted in a 50% inhibition in DCA formation. The inhibitors of cytochromes P-450, SKF 525-A and 1-aminobenzotriazol, produced 85 and 70% inhibitions of DCA formation, respectively. When alcohol dehydrogenase and NADH were added to microsomal incubations, two TCP-related alcohols, 1,3-dichloro-2-propanol and 2,3-dichloropropanol, were formed. These alcohols are products of the initial microsomal metabolites, DCA and 2,3-dichloropropanal. [14C]TCP equivalents bound covalently to rat hepatic microsomal protein. This binding was increased 8-fold when hepatic microsomes from phenobarbital pretreated rats were used. The addition of either glutathione or N-acetylcysteine to the incubations completely inhibited this binding. In the presence of N-acetylcysteine, 1,3-(2-propanone)-bis-S-(N-acetylcysteine) (PDM) was the only N-acetylcysteine conjugate detected. It represented 87% of TCP microsomal metabolism. The formation of PDM implicates DCA as the major microsomal protein-binding metabolite of TCP. The formation of DCA, a direct-acting mutagen, may be responsible for the mutagenicity of TCP in systems using rat hepatic microsomes ...
来源:Hazardous Substances Data Bank (HSDB)
代谢
1,2,3-三氯丙烷内源性地形成反应性中间体,在1,2,3-三氯丙烷的器官毒性、基因毒性和致癌作用中起着关键作用。假设的烷基化物种的形成与肝脏和肾脏以及靶组织中致癌作用的目标组织中共价结合的放射性物质的存在的观点是一致的,在给小鼠灌胃[(14)C]1,2,3-三氯丙烷后。在小鼠的贲门和肝脏以及在大鼠的肾脏、肝脏、胰腺、舌头和贲门中发现了显著的DNA加合物水平(以1,2,3-三氯丙烷的(14)C当量表示)。在肝脏中代谢1,2,3-三氯丙烷时现场形成的亚磺酸离子和在肾脏中处理1,2,3-三氯丙烷的谷胱甘肽结合物被赋予了主要作用,导致肝脏和肾脏的损伤。
The endogenous formation of reactive intermediates from 1,2,3-trichloropropane plays a crucial role in the organotoxic, genotoxic, and carcinogenic action of 1,2,3-trichloropropane. The postulated formation of alkylating species is consistent with the presence of covalently bound radioactivity in liver and kidney and in target tissues of carcinogenic action after gavage administration of [(14)C]1,2,3-trichloropropane. Significant DNA adduct levels (as (14)C equivalents of 1,2,3-trichloropropane) were found in forestomach and liver in mice and in kidney, liver, pancreas, tongue, and forestomach in rats. A predominant role was assigned to episulfonium ions formed in situ during the metabolism of 1,2,3-trichloropropane in the liver and from processing of GSH conjugates of 1,2,3-trichloropropane in the kidney, leading to hepatotoxic and nephrotoxic lesions.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
识别和使用:1,2,3-三氯丙烷(TCP)是一种无色液体,具有刺鼻的、令人不快的气味。它被用作合成其他化学品(例如,杀虫剂)的中间体,并在生产聚合物(如多硫化物和六氟丙烯)中作为交联剂。以前的使用包括作为疏水性化合物和树脂的溶剂,用作油漆和清漆的去除剂,以及作为脱脂剂。
人类暴露和毒性:在100 ppm的浓度下,1,2,3-三氯丙烷的蒸气被实验对象认为由于眼睛和喉咙刺激以及令人不快的气味而无法接受。注意到在50 ppm时,1,2,3-TCP的蒸气是刺鼻的。1,2,3-TCP的急性/亚慢性神经毒性效应包括人类的中枢神经系统损伤。已有零星报道称人类因1,2,3-TCP导致的中毒性肝损伤。在体外微核试验中,与同期对照组相比,1,2,3-TCP未表现出致突变活性。在碱性单细胞凝胶电泳试验(彗星试验)中,评估了1,2,3-TCP在有无S9混合物的孤立人类淋巴ocytes中的DNA断裂能力和细胞毒性。1,2,3-TCP诱导了DNA断裂。1,2,3-TCP被怀疑是人类的致癌物。
动物研究:将0.5 mL的1,2,3-TCP应用于兔子的完整皮肤,半封闭条件下4小时,封闭条件下24小时,产生了轻微的可逆性刺激。与这些测试结果相比,另一项研究根据Draize测试中兔子完整和磨损皮肤上的刺激分数将1,2,3-TCP归类为严重刺激物。在兔子眼中滴入0.1 mL未稀释的1,2,3-TCP后,观察到轻微到中度的刺激,所有效果在2-7天内可逆。大鼠和小鼠的中毒症状包括虚脱、活动减少、共济失调、镇静、呼吸困难、抽搐、流泪、流涎、眼睛和鼻粘膜刺激以及肝脏和肾脏损伤。立即呼吸抑制是死亡的常见原因。延迟死亡(7-10天后)归因于肝脏损伤。三只狗经口给予1,2,3-TCP后,1-2小时内出现中枢神经系统抑制,并在1-2天内死亡。连续7天吸入0.4和0.8 mg/立方米的1,2,3-TCP在大鼠肺中引起出血,支气管、支气管和肺泡上皮脱落和破坏。给予113或149 mg/kg的1,2,3-TCP饮用水的 rats for 13 weeks developed renal pyknosis, glomerular adhesions, and accumulated protein in the tubular lumen. INTermitteNT 4-hour exposures for 11 days at 3 ppm of 1,2,3-TCP caused a decreased thickness of olfactory epithelium in rats. 在10 ppm或更高浓度下,在大鼠中发现了嗅上皮的变性,而在小鼠中在10到13 ppm浓度下发现了炎症和嗅上皮厚度的减少,在更高浓度下出现变性。在长期动物研究中测试的140种致癌化学物质中,1,2,3-TCP是导致小鼠子宫肿瘤的七种化学物质之一(自发肿瘤率为0.3%)。NTP对1,2,3-TCP进行了为期2年的毒理学和致癌性研究。1,2,3-TCP在大小鼠的多个部位诱导了良性或恶性肿瘤。怀孕大鼠在整个着床和器官形成期间通过腹腔注射给予15剂37 mg/kg的1,2,3-TCP,后代没有出现胎毒或致畸效应,但母体毒性明显。雄性大鼠口服五剂80 mg/kg/天,没有出现睾丸毒性的证据。在一项单代繁殖研究中,后代指数未受到1,2,3-TCP处理的影响。雄性大鼠经胃管给予1,2,3-TCP,剂量为125 mg/kg体重/天,持续120天,结果显示相对睾丸重量显著增加,相对附睾重量显著减少。然而,没有观察到对精子计数或精子形态的影响,也没有观察到睾丸或附睾的组织形态学变化。在处理过的雌性大鼠与未处理过的雄性大鼠交配的后代中,观察到生存率的损害。1,2,3-TCP在含有代谢活化的沙门氏菌鼠伤寒菌株TA 97、TA 100和TA 1535以及E. coli WP2 uvr A的细菌测试系统中具有致突变性。1,2,3-TCP未在原代大鼠肝细胞中诱导非计划性DNA合成。它在L5178Y小鼠淋巴瘤细胞的胸腺嘧啶激酶位点上诱导基因突变,在中国仓鼠V79和卵巢细胞中诱导姐妹染色单体
IDENTIFICATION AND USE: 1,2,3-Trichloropropane (TCP) is a colorless liquid with a acrid, unpleasant. It is used as an intermediate in the synthesis of other chemicals (e.g., pesticides) and as a crosslinking agent in the production of polymers, such as polysulfides and hexafluoropropylene. Former uses include a solvent for hydrophobic compounds and resins, as a paint and varnish remover, and as a degreasing agent. HUMAN EXPOSURE AND TOXICITY: Vapors of 1,2,3-trichloropropane were found to be objectionable to subjects at a concentration of 100 ppm because of eye and throat irritation and unpleasant odor. It was noted that vapors of 1,2,3-TCP were acrid at 50 ppm. Acute/subchronic neurotoxic effects of 1,2,3-TCP include CNS damage in humans. Sporadic cases of toxic hepatic injury from 1,2,3-TCP in humans have been reported. In an in vitro micronucleus assay, 1,2,3-TCP did not exhibit mutagenic activity compared to the concurrent control. The DNA breakage capacity and the cytotoxicity was assessed in the alkaline single cell gel electrophoresis test (comet assay) with and without S9-mix in isolated human lymphocytes. 1,2,3-TCP induced DNA breakage. 1,2,3-TCP is a suspected human carcinogen. ANIMAL STUDIES: A dose of 0.5 mL of 1,2,3-TCP applied to the intact skin of rabbits for 4 hr under semi-occlusive and for 24 hr under occlusive dressings produced mild reversible irritation. In contrast to these test results, another study classified 1,2,3-TCP as severely irritating, based on irritation scores from a Draize test on intact and abraded skin of rabbits. After instillation of 0.1 mL of undiluted 1,2,3-TCP, mild to moderate irritation of rabbit eyes was observed, with all effects being reversible within 2-7 days. Symptoms of intoxication in rats and mice included prostration, hypoactivity, ataxia, sedation, dyspnea, convulsions, lacrimation, salivation, irritation of the mucosa of eyes and nose, and liver and kidney damage. Immediate respiratory depression was a frequent cause of death. Delayed deaths (after 7-10 days) were ascribed to liver damage. Three dogs that received intragastic administration of 1,2,3-TCP had CNS depression, within 1-2 hours, and died within 1-2 days. Continuous inhalation of 0.4 and 0.8 mg/cu m of 1,2,3-TCP for 7 days caused hemorrhages in rat lungs with desquamation and destruction of bronchial, bronchiolar & alveolar epithelium. Rats given 113 or 149 mg/kg 1,2,3-TCP in drinking water for 13 weeks developed renal pyknosis, glomerular adhesions, and accumulated protein in the tubular lumen. Intermittent 4-hour exposures for 11 days at 3 ppm of 1,2,3-TCP caused a decreased thickness of olfactory epithelium in rats. A degeneration of the olfactory epithelium was found in rats at 10 ppm or higher concentrations, and inflammation and decreased thickness of the olfactory epithelium were found in mice at 10 to 13 ppm with degeneration at higher concentrations. Among 140 carcinogenic chemicals tested in long-term animal studies, 1,2,3-TCP was among the seven producing uterine neoplasms in mice (spontaneous tumor rate 0.3%). 1,2,3-TCP was evaluated in 2-year toxicology and carcinogenesis studies by the NTP. 1,2,3-TCP induced benign and/or malignant neoplasms at multiple sites in both rats and mice. 15 daily doses of 37 mg/kg 1,2,3-TCP were given by intraperitoneal injection to pregnant rats throughout implantation and organogenesis; there were no fetotoxic or teratogenic effects in the offspring, but maternal toxicity was evident. Male rats given five oral doses of 80 mg/kg/day showed no evidence of testicular toxicity. In a one-generation reproduction study, progeny indices were unaffected by treatment with 1,2,3-TCP. Male rats administered 1,2,3-TCP by gavage at a dosage of 125 mg/kg body weight per day for 120 days showed significant increases in relative testes weights and a significant decrease in relative epididymis weight. However, no effects on sperm count or sperm morphology and no histomorphological changes of testes or epididymis were observed. Impairment of survival rates was observed among pups of treated females that had been mated with untreated males. 1,2,3-TCP is mutagenic in bacterial test systems with Salmonella typhimurium strains TA 97, TA 100, and TA 1535 and E. coli WP2 uvr A in the presence of metabolic activation. 1,2,3-TCP did not induce unscheduled DNA synthesis in primary rat hepatocytes. It induced gene mutation at the thymidine kinase locus in L5178Y mouse lymphoma cells, sister chromatid exchange in Chinese hamster V79 and ovary cells and chromosomal aberrations in Chinese hamster ovary cells, only in the presence of metabolic activation. Additionally, 1,2,3-TCP enhanced the transformation of Syrian hamster embryo cells by simian adenovirus SA7.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
评估:对于1,2,3-三氯丙烷对人类致癌性的证据不足。对于1,2,3-三氯丙烷对实验动物致癌性的证据充分。总体评估:1,2,3-三氯丙烷可能对人类致癌(2A组)。在进行总体评估时,工作组考虑了以下证据:(1)1,2,3-三氯丙烷在小鼠和大鼠的多个部位和高发病率上引起肿瘤。(2)1,2,3-三氯丙烷的代谢在人类和啮齿类微粒体中质上相似。(3)1,2,3-三氯丙烷对细菌和培养的哺乳动物细胞具有诱变性,并能够与体内处理的动物DNA结合。
Evaluation: There is inadequate evidence in humans for the carcinogenicity of 1,2,3-trichloropropane. There is sufficient evidence in experimental animals for the carcinogenicity of 1,2,3-trichloropropane. Overall evaluation: 1,2,3-Trichloropropane is probably carcinogenic to humans (Group 2A). In making the overall evaluation, the working group took into account the following evidence: (1) 1,2,3-Trichloropropane causes tumors at multiple sites and at high incidence in mice and rats. (2) The metabolism of 1,2,3-trichloropropane is qualitatively similar in human and rodent microsomes. (3) 1,2,3-Trichloropropane is mutagenic to bacteria and to cultured mammalian cells and binds to the DNA of animals treated in vivo.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
A2:疑似人类致癌物。
A2: Suspected human carcinogen.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
1,2,3-三氯丙烷根据实验动物研究的充分致癌性证据,合理预期为人类致癌物。
1,2,3-Trichloropropane is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
国际癌症研究机构致癌物:1,2,3-三氯丙烷
IARC Carcinogenic Agent:1,2,3-Trichloropropane
来源:International Agency for Research on Cancer (IARC)
吸收、分配和排泄
为了研究1,2,3-三氯丙烷(TCP)的处置情况,将[(14)C]-TCP静脉注射到雄性Fischer 344大鼠中。在不同时间点给药后,测定组织和排泄物中未改变的TCP和总放射性标记。该化合物分布和消除迅速...在15分钟内,脂肪组织积累了剂量的37%,并且在4小时内比任何其他组织保留更多的剂量;在4小时内,脂肪组织中大部分(69%)的放射性标记是未改变的TCP。4小时后,肝脏含有剂量的最大部分,主要是代谢物。因此,TCP从脂肪组织中消失,而代谢物出现在肝脏和其他组织中。排泄在24小时内几乎完成(剂量的90%),主要通过尿液(剂量的47%)。未改变的TCP唯一的排泄途径是通过呼气(剂量的5%)。此外,剂量的25%以二氧化碳形式呼出。还有许多其他代谢物,没有一个占剂量的10%以上。非挥发性代谢物的寿命比母体化合物长。基于高水溶性、与2,4-二硝基氟苯反应以及糖苷酸处理大鼠胆汁中放射性标记减少,建议谷胱甘肽结合是TCP的一个重要代谢途径...
To investigate the disposition of 1,2,3-trichloropropane (TCP), [(14)C]-TCP was administered iv to male Fischer 344 rats. Unchanged TCP and total radiolabel were determined in tissues and excreta at varying intervals after administration. The compound was distributed and eliminated rapidly ... Adipose tissue accumulated 37% of the dose within 15 min and retained more of the dose than any other tissue until 4 hr; most (69%) of the radiolabel in adipose tissue through 4 hr was unchanged TCP. After 4 hr, the liver contained the largest fraction of the dose, primarily as metabolites. Thus TCP disappeared from adipose tissue while metabolites appeared in liver and other tissues. Excretion was nearly complete (90% of the dose) in 24 hr and was predominantly via the urine (47% of the dose). Expiration was the only route by which unchanged TCP (5% of the dose) was excreted. In addition, 25% of the dose was expired as carbon dioxide. There were numerous other metabolites, none accounting for more than 10% of the dose. Nonvolatile metabolites were longer lived than the parent compound. On the basis of high water solubility, reaction with 2,4-dinitrofluorobenzene, and diminished radiolabel in bile of glycidol-treated rats, glutathione conjugation is suggested as an important metabolic route for TCP ...
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
... 在对F344大鼠(30 mg/kg)和B6C3F1雄性小鼠(30和60 mg/kg)进行急性口服暴露后,1,2,3-三氯丙烷被迅速吸收、代谢并排出。1,2,3-三氯丙烷的主要排泄途径是通过尿液。在给药后60小时,大鼠通过此途径排出了50%,而小鼠排出了65%的给药剂量。通过呼吸排出二氧化碳((14)C02)和在粪便中排泄各占60小时大鼠总剂量的20%,在小鼠中分别占20%和15%。在大鼠排泄1,2,3-三氯丙烷派生物活性的能力上,没有观察到明显的性别差异。在60小时时,1,2,3-三氯丙烷派生物活性在大鼠和雄性小鼠的肝脏、肾脏和前胃中含量最高。雄性小鼠消除1,2,3-三氯丙烷派生物活性的速度比大鼠快,在给药后60小时,小鼠组织中的放射性活性浓度较低。...
... Following acute oral exposure of male and female F344 rats (30 mg/kg) and male B6C3F1 mice (30 and 60 mg/kg), 1,2,3-trichloropropane was rapidly absorbed, metabolized, and excreted. The major route of excretion of 1,2,3-trichloropropane was in the urine. By 60 hr postdosing, rats had excreted 50% and mice 65% of the administered dose by this route. Exhalation as (14)C02 and excretion in the feces each accounted for 20% of the total dose in 60 hr rats and 20 and 15%, respectively, in mice. No apparent sex-related differences were observed in the ability of the rats to excrete 1,2,3-trichloropropane derived radioactivity. At 60 hr, 1,2,3-trichloropropane derived radioactivity was most concentrated in the liver, kidney, and forestomach in both rats and male mice. Male mice eliminated 1,2,3-trichloropropane derived radioactivity more rapidly than rats and lower concentrations of radioactivity were found in tissues 60 hr after dosing in mice. ...
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
治疗六小时后,最高放射性活性在大鼠的前胃、小肠和雄性大鼠的脂肪组织中被发现。24小时后,最高放射性活性仍然在前胃中发现,但也在大肠中发现。在雄性和雌性大鼠的脂肪组织中没有观察到增加的放射性活性。60小时后,放射性活性在前胃(最高值)、肝脏和肾脏中被检测到。将30或60毫克的[(14)C] 1,2,3-TCP注射到B6C3F1小鼠中,60小时后在前胃、肝脏和肾脏中发现了高放射性活性。1,2,3-TCP在组织中的分布与剂量无关。
Six hours post-treatment the highest radioactivity was found in the forestomach, in the small intestine and in adipose tissue of male rats. After 24 hours the highest radioactivity was still found in the forestomach, but also in the large intestine. No increased radioactivity was observed in adipose tissue of male and female rats. After 60 hours radioactivity was detected in the forestomach (highest value), and in the liver and kidney. The injection of 30 or 60 mg [(14)C] 1,2,3-TCP to B6C3F1 mice resulted in high radioactivity in the forestomach, the liver and kidney 60 hours post-treatment. 1,2,3-TCP distribution in tissues was not dose dependent.
来源:Hazardous Substances Data Bank (HSDB)
安全信息
-
职业暴露等级:A
-
职业暴露限值:TWA: 10 ppm (60 mg/m3) [skin]
-
TSCA:Yes
-
危险等级:6.1
-
立即威胁生命和健康浓度:100 ppm
-
危险品标志:T
-
安全说明:S36,S36/37,S37,S45,S53
-
危险类别码:R60,R20/21/22,R45
-
WGK Germany:3
-
海关编码:29031980
-
危险品运输编号:UN 2810 6.1/PG 3
-
RTECS号:TZ9275000
-
包装等级:III
-
危险类别:6.1
-
储存条件:储存注意事项:应储存在阴凉、通风良好的库房中,并远离火源和热源。保持容器密封,切勿与其他氧化剂、碱类或食用化学品混存。需配备相应种类和数量的消防器材。储存区应备有泄漏应急处理设备及合适的收容材料。
SDS
| 国标编号: | 61559 |
| CAS: | 96-18-4 |
| 中文名称: | 1,2,3-三氯丙烷 |
| 英文名称: | 1,2,3-trichloropropane |
| 别 名: | |
| 分子式: | C 3 H 5 Cl 3 ;CH 2 ClCHClCH 2 Cl |
| 分子量: | 147.44 |
| 熔 点: | -14.7℃ 沸点:156.2? |
| 密 度: | 相对密度(水=1)1.39 |
| 蒸汽压: | 82℃ |
| 溶解性: | 微溶于水,溶于油类 |
| 稳定性: | 稳定 |
| 外观与性状: | 无色液体 |
| 危险标记: | 14(有毒品) |
| 用 途: | 用作溶剂 |
2、对环境的影响
该物质对环境可能有危害,在地下水中有蓄积作用。
一、健康危害
侵入途径:吸入、食入、经皮吸收。
健康危害:本品具有麻醉作用。急性接触时,有较强的呼吸道及局部刺激作用。经皮吸收亦可引起中毒。
二、毒理学资料及环境行为
急性毒性:LD50320mg/kg(大鼠经口);1770mg/kg(兔经皮);LC503400mg/m3,2小时(小鼠吸入)
危险特性:与强氧化剂接触可发生化学反应。受热易分解,燃烧时产生有毒的氯化物气体。遇潮湿空气能水解生成微量的氯化氢,光照亦能促进水解而对金属的腐蚀性增强。
燃烧(分解)产物:一氧化碳、二氧化碳、氯化氢。
3、现场应急监测方法
4、实验室监测方法
监测方法 来源 类别
气相色谱法;
色谱/质谱法 《固体废弃物试验与分析评价手册》中国环境监测总站等译 固体废弃物
色谱/质谱法 《水和废水标准检验法》19版译文,江苏省环境监测中心 水和废水
色谱/质谱法 美国EPA524.2方法 水质
空气中样品的测定:样品用活性炭吸附、二硫化碳洗脱,再用气相色谱法测定(NIOSH法)
5、环境标准
前苏联 车间空气中有害物质的最高容许浓度 2mg/m3
前苏联(1978)生活饮用水和娱乐用水水体中有害物质的最大允许浓度 0.07mg/L
6、应急处理处置方法
一、泄漏应急处理
迅速撤离泄漏污染区人员至安全区,并进行隔离,严格限制出入。切断火源。建议应急处理人员戴自给正压式呼吸器,穿防毒服。尽可能切断泄漏源,防止进入下水道、排洪沟等限制性空间。小量泄漏:用砂土、蛭石或其它惰性材料吸收。大量泄漏:构筑围堤或挖坑收容;用泡沫覆盖,降低蒸气灾害。用防爆泵转移至槽车或专用收集器内,回收或运至废物处理场所处置。
废弃物处置方法:建议用焚烧法处置。废料同其它燃料混合后焚烧。燃烧要充分,防止生成光气。焚烧炉排气中的卤化氢通过酸洗涤器除去。
二、防护措施
呼吸系统防护:空气中浓度超标时,应选择佩带自吸过滤式防毒面具(半面罩)。紧急事态抢救或撤离时,佩戴氧气呼吸器。
眼睛防护:戴安全防护眼镜。
身体防护:穿透气型防毒服。
手防护:戴防化学品手套。
其它:工作现场禁止吸烟、进食和饮水。工作毕,沐浴更衣。单独存放被毒物污染的衣服,洗后备用。
三、急救措施
皮肤接触:脱去被子污染的衣着,用肥皂水和清水彻底冲洗皮肤。就医。
眼睛接触:提起眼睑,用流动清水或生理盐水冲洗。
吸入:迅速脱离现场至空气新鲜处。保持呼吸道通畅。如呼吸困难,给输氧。如呼吸停止,立即进行人工呼吸。就医。
食入:饮足量温水,催吐,就医。
灭火方法:消防人员须佩戴防毒面具、穿全身消防服。喷水保持火场容器冷却,直至灭火结束。灭火剂:雾状水、泡沫、二氧化碳、砂土。
制备方法与用途
三氯丙烷
基本信息
三氯丙烷是一种重要的有机合成中间体,用于制备甘油、三氯丙烯、丙炔醇、三乙酸甘油酯、四氯丙烯、2-氯丙胺、多氯丙烷、2,4-二甲基-3-乙酰基呋喃;也是生产农药原料,可用于生产农药矮壮素和燕麦敌。此外,它还是一种较好的溶剂,用于清除金属表面的涂料及油漆,也可作为油脂、蜡、树脂及氯化橡胶的溶剂。三氯丙烷与碱金属多硫化物、双二氯乙烯基缩甲醛缩聚生成ST型聚硫橡胶,同时也用作色谱分析标准物质。
性质与用途- 毒性与危险情况
-
不相容性
- 与活泼金属、强碱及强氧化剂均不相容。
- 有害影响和中毒症状
操作时应穿戴适当的工作服,防止皮肤反复或长时间接触。戴防护眼镜以避免眼的直接接触。如有湿透或污染的工作服,立即更换,并在操作现场备置安全信号指示器和洗眼剂。
医学监护就业前及定期体检时应注意检查侵害部位。
测定方法空气中三氯丙烷的测定:用活性炭吸附、CS₂处理,通过气相色谱法分析。
贮存使用塑料桶或铁桶盛装,并防止机械性损坏。应存放在阴凉、通风和干燥的地方,远离火源。
运输要求三氯丙烷属有机易燃有毒品。危规号:84007;运输时容器上须标有“易燃有毒”标记。
废弃物处理建议焚烧更为可取,与其它易燃燃料混合后焚烧更佳。必须注意完全燃烧以防止光气产生,且应配备酸洗涤器以除去产生的氢卤酸。
化学性质 类别与毒性分级- 类别:有毒物品
- 毒性分级:高毒
-
急性毒性
- 大鼠口服LD₅₀: 140 毫克/公斤;小鼠口服LD₅₀: 369毫克/公斤。
-
刺激数据
- 皮肤接触兔子700毫克,轻度;眼睛接触兔子140毫克,重度。
- 与空气混合可以爆炸
- 受热分解并燃烧时产生有毒氯化物气体
- 与氧化剂反应
TWA:300毫克/立方米;STEL:450毫克/立方米。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1,2-二氯丙烷 1,2-Dichloropropane 78-87-5 C3H6Cl2 112.987 1,3-二氯丙烷 1,3-Dichloropropane 142-28-9 C3H6Cl2 112.987 1,1,2-三氯丙烷 1,1,2-trichloropropane 598-77-6 C3H5Cl3 147.432 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 四氯丙烷 1,2,2,3-tetrachloropropane 13116-53-5 C3H4Cl4 181.877 四氯丙烷 1,1,2,3-tetrachloropropane 18495-30-2 C3H4Cl4 181.877 1,1,2,3,3-五氯丙烷 1,1,2,3,3-pentachloropropane 15104-61-7 C3H3Cl5 216.322
反应信息
-
作为反应物:描述:1,2,3-三氯丙烷 在 sodium nitrite 作用下, 100.0 ℃ 、100.0 kPa 条件下, 反应 24.0h, 生成 1,3-Dichloro-2-nitropropane参考文献:名称:一种溴硝醇的制备方法摘要:本发明公开了一种溴硝醇的制备方法,其特征在于,包括如下制备步骤:将化合物1A与硝化试剂进行反应,得到化合物2A;将上述的化合物2A与水进行水解反应,再与溴化试剂进行反应,即得溴硝醇粗品,经过纯化后,得到溴硝醇精品。本发明采用价格较低的试剂作为起始原料,经过硝化、水解、溴化等反应,得到最终产品,每步的反应条件温和,得到的溴硝醇收率高,能够大幅降低成本。公开号:CN112679359B
-
作为产物:描述:参考文献:名称:烯烃氯化的极性反应常数。氯离子和溴离子的过渡态相似和不同。摘要:在25℃下通过直接动力学方法在甲醇中获得的用于将游离氯加成到烯烃中的极性反应常数ϱ * cl 2为-2.9,而ϱ * Br 2为-3.1。从溴化到氯化的变化与大的反应性增加但选择性的小下降有关。根据Harmond假设以及在卤化过渡态中电荷分布对桥连原子的依赖性来讨论此结果。DOI:10.1016/s0040-4039(00)87590-5
-
作为试剂:描述:2-苯基吡啶 在 1,2,3-三氯丙烷 、 氧气 、 copper diacetate 、 二氧化氮 作用下, 反应 16.0h, 以85%的产率得到2-(2,6-dichlorophenyl)pyridine参考文献:名称:铜介导的(杂)芳烃螯合辅助邻硝化摘要:首次开发了一种新型的铜介导的(杂)芳烃螯合辅助邻硝基甲烷硝化反应,该反应使用双氧作为末端氧化剂,并使用1,2,3-TCP作为溶剂,导致硝基芳烃的合成极好的区域选择性和高收率。机理研究表明,该机理涉及一个四中心过渡态,同时裂解了2-芳基吡啶配位的铜(II)络合物上的硝酸根阴离子的邻位CH键和N-O键。DOI:10.1021/ol2028288
文献信息
-
ORGANIC ELECTROLUMINESCENCE DEVICE AND ANTHRACENE DERIVATIVE申请人:IKEDA Hidetsugu公开号:US20110034744A1公开(公告)日:2011-02-10An anthracene derivative having a specific asymmetric structure is provided. The asymmetric anthracenes are useful in an organic electroluminescence device and exhibit efficient light emission and a long performance lifetime.
-
PHENANTHRENE DERIVATIVE, AND MATERIAL FOR ORGANIC EL ELEMENT申请人:Kawamura Masahiro公开号:US20100331585A1公开(公告)日:2010-12-30A phenanthrene derivative is represented by a formula (1) below. In the formula (1), Ar 1 and Ar 2 each represent an aromatic hydrocarbon ring group having 6 to 18 carbon atoms for forming the ring. The aromatic hydrocarbon ring group contains none of anthracene skeleton, pyrene skeleton, aceanthrylene skeleton and naphthacene skeleton. R 1 represents a substituent, the number of which may be 0, 1 or more. R 1 may be bonded in any position of the phenanthrene skeleton. n and m each represent an integer of 1 to 3. k represents an integer of 0 to 8.
-
AROMATIC AMINE DERIVATIVE AND ORGANIC ELECTROLUMINESCENT DEVICE USING SAME申请人:Idemitsu Kosan Co., Ltd.公开号:EP1997799A1公开(公告)日:2008-12-03Provided are a novel aromatic amine derivative represented by the following general formula (1) and an organic electroluminescent device including an organic thin film formed of one or a plurality of layers including at least a light emitting layer interposed between a cathode and an anode, in which at least one layer of the organic thin film contains the aromatic amine derivative alone or as a component of a mixture, which contributes to suppressing molecular crystallization and improving yield in the production of an organic electroluminescent device, whereby an organic electroluminescent device having a long lifetime can be obtained, and the aromatic amine derivative is capable of realizing the organic electroluminescent device: where: R1 represents an aryl group or the like; a represents an integer of 0 to 4; b represents an integer of 1 to 3; and at least one of Ar1 to Ar4 represents a group represented by the following general formula (2): where Ar5 represents a fused aromatic ring group and Ar6 represents an aryl group or an aromatic heterocyclic group, where remaining groups of Ar1 to Ar4, none of which is represented by the general formula (2), each independently represent an aryl group or an aromatic heterocyclic group.提供了一种由以下一般式(1)表示的新型芳香胺衍生物,以及包括由至少一个或多个层次组成的有机薄膜的有机电致发光装置,其中有机薄膜的至少一层包含仅芳香胺衍生物或作为混合物成分的芳香胺衍生物,有助于抑制分子结晶并提高有机电致发光装置的产量,从而可以获得寿命长的有机电致发光装置,且该芳香胺衍生物能够实现该有机电致发光装置: 其中:R1代表芳基或类似物;a代表0到4的整数;b代表1到3的整数;Ar1至Ar4中至少一个代表由以下一般式(2)表示的基团: 其中Ar5代表融合芳香环基团,Ar6代表芳基或芳香杂环基团, Ar1至Ar4的其余基团,其中没有一个被一般式(2)表示,各自独立地代表芳基或芳香杂环基团。
-
PROCESS FOR THE PREPARATION OF 2,3,3,3 TETRAFLUOROPROPENE申请人:Wendlinger Laurent公开号:US20130267740A1公开(公告)日:2013-10-10The present invention provides a process for preparing 2,3,3,3-tetrafluoropropene, comprising the following steps: (a) catalytic reaction of 1,1,1,2,3-pentachloropropane and/or 1,1,2,2,3-pentachloropropane with HF into product 2-chloro-3,3,3-trifluoropropene; (b) catalytic reaction of the thus-obtained 2-chloro-3,3,3-trifluoropropene into 2,3,3,3-tetrafluoropropene.本发明提供了一种制备2,3,3,3-四氟丙烯的方法,包括以下步骤:(a)将1,1,1,2,3-五氯丙烷和/或1,1,2,2,3-五氯丙烷与氢氟酸催化反应,生成产物2-氯-3,3,3-三氟丙烯;(b)将所得的2-氯-3,3,3-三氟丙烯进行催化反应,得到2,3,3,3-四氟丙烯。
-
[EN] PROCESS FOR PREPARING 1,1,2,3-TETRACHLOROPROPENE<br/>[FR] PROCÉDÉ DE SYNTHÈSE DE 1,1,2,3-TÉTRACHLOROPROPÈNE申请人:DAIKIN IND LTD公开号:WO2011065574A1公开(公告)日:2011-06-03The present invention provides a process for preparing 1,1,2,3-tetrachloropropene, including heating 1,1,1,2,3-pentachloropropane in a gas phase in the absence of a catalyst to carry out a dehydrochlorination reaction. According to the process of the present invention, 1,1,2,3-tetrachloropropene (HCC-1230xa) can be efficiently produced by a simple and economically advantageous method that is suitable for industrial-scale production.本发明提供了一种制备1,1,2,3-四氯丙烯的方法,包括在无催化剂存在的气相中加热1,1,1,2,3-五氯丙烷以进行脱氯化反应。根据本发明的方法,可以通过一种简单且经济优势的方法高效生产1,1,2,3-四氯丙烯(HCC-1230xa),适用于工业规模生产。
表征谱图
-
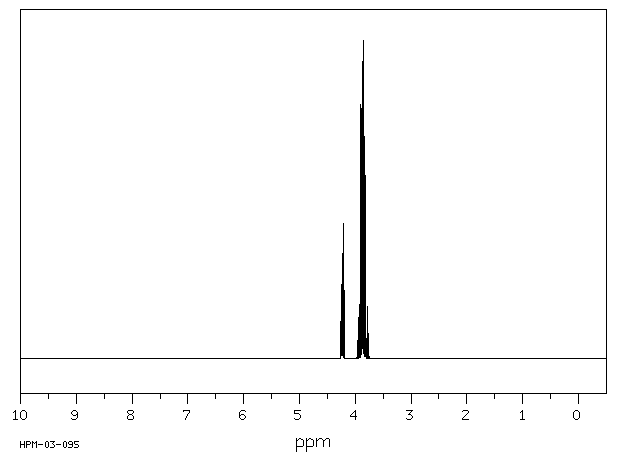
氢谱1HNMR
-
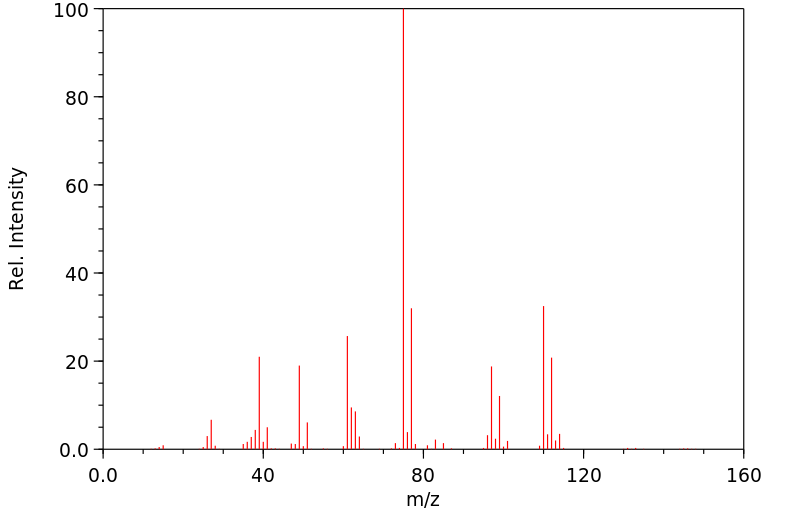
质谱MS
-
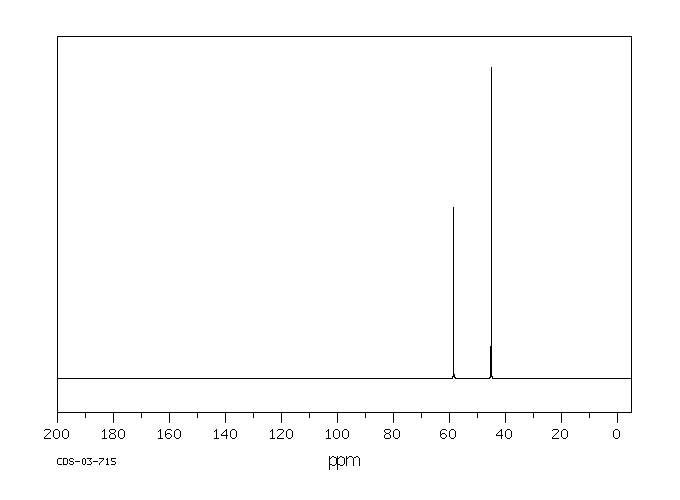
碳谱13CNMR
-
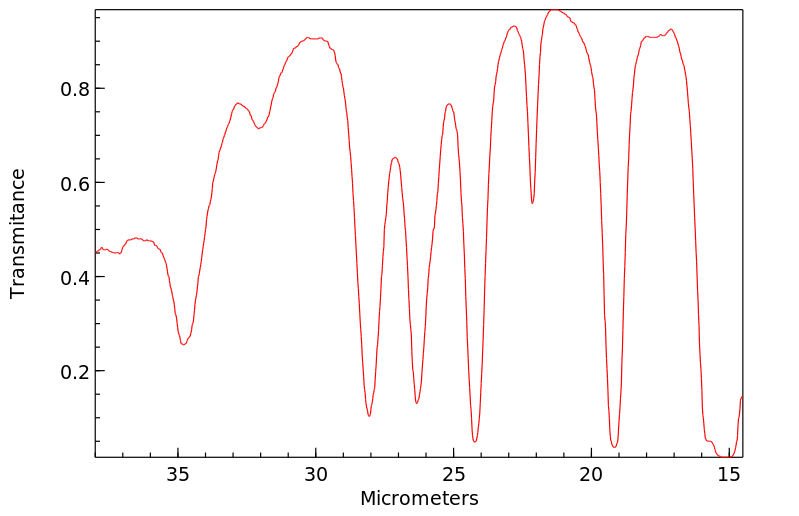
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式1,4-二氯-2-甲基-2-丁烯
顺式1,1,1,5-四氯-4-甲基-3-戊烯
顺式-7-甲基环庚-2-烯基氯
顺式-4-甲基环庚-2-烯基氯
顺式-1-氨基-4-氯-2-丁烯
顺式-1,4-二氯-2-丁烯
顺-6-氯-2-己烯
顺-4-氯-2-丁烯胺盐酸盐
锡烷,二(4-氯丁基)羰基-
锡烷,三氯(2-乙烯基壬基)-
重氮乙酰氯
辛基癸基二甲基氯化铵
聚乙烯胺
羟肟基乙酰氯
磷亚胺三氯化,[1,2,2,2-四氯-1-(三氯甲基)乙基]-
硫代氯甲酸-O-辛酯
癸醛,2,2-二氯-
甲醛与氨和氯乙烷的聚合物
甲基(2E)-2-(3-氯-2-丁烷亚基)肼羧酸酯
环己烷,(氯甲基)-
环丙烷,2-丁基-1-氯-1-(1-戊炔基)-,顺-
环丙烷,1,2-二溴-3,3-二氯-1,2-二丙基-,反-
环丙烷,1,1-二溴-2,3-二氯-2,3-二乙基-,反-
环丙烷,1,1-二氯-3-(氯甲基)-2,2-二甲基-
环丙烷,1,1,2,3-四氯-2,3-二甲基-,反-
环丙基甲基氯
环丁基氯
特比萘芬杂质17
溴代二氯丁烷
油酰氯
油酰氯
水合2-氯乙醛
氯螺戊烷
氯磺酸-(2,3-二氯丙酯)
氯甲醇
氯甲氧基
氯甲基自由基
氯甲基环丁烷
氯甲基氯磺酸酯
氯甲基二氯甲基醚
氯甲基(甲基)次磷酰氯
氯环辛烷
氯环癸烷
氯环庚烷
氯环丙烷
氯十七烷
氯化链烷烃
氯化环十二烷
氯化新戊烷
氯代环戊烷