1-(3-三氟甲基苯基)咪唑 | 25371-97-5
中文名称
1-(3-三氟甲基苯基)咪唑
中文别名
——
英文名称
1-(3-(trifluoromethyl)phenyl)-1Himidazole
英文别名
1-(3-Trifluoromethylphenyl)imidazole;1-[3-(trifluoromethyl)phenyl]imidazole
CAS
25371-97-5
化学式
C10H7F3N2
mdl
MFCD00060487
分子量
212.174
InChiKey
KZVUPVJLOACZIL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:70 °C
-
密度:1,32 g/cm3
计算性质
-
辛醇/水分配系数(LogP):2.6
-
重原子数:15
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.1
-
拓扑面积:17.8
-
氢给体数:0
-
氢受体数:4
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
危险类别码:R36/37/38
-
海关编码:2933290090
-
安全说明:S26,S36/37/39
SDS
反应信息
-
作为反应物:描述:1-(3-三氟甲基苯基)咪唑 在 silver(l) oxide 作用下, 以 四氢呋喃 、 乙二醇乙醚 为溶剂, 反应 24.0h, 生成 2-(2,4-difluorobenzene-6-id-1-yl)pyridine;iridium(3+);1-methyl-3-[3-(trifluoromethyl)benzene-6-id-1-yl]-2H-imidazol-3-ium-2-ide参考文献:名称:N-Heterocyclic Carbenes: Versatile Second Cyclometalated Ligands for Neutral Iridium(III) Heteroleptic Complexes摘要:With 2-(2,4-difluorophenyl)pyridine (dfppy) as the first cyclometalated ligand and different monoanionic N-heterocyclic carbenes (NHCs) as the second cyclometalated ligands, 16 blue or greenish-blue neutral iridium(III) phosphorescent complexes, (dfppy)(2)Ir(NHC), were synthesized efficiently. The obtained Ir(III) complexes display typical phosphorescence of 455-485 nm with quantum yields up to 0.73. By modifying the phenyl moiety in the NHCs with electron-withdrawing substituents (e.g., -F or -CF3) or replacing it with N-heteroaromatic rings (pyridine or pyrimidine), the HOMO-LUMO gaps are broadened, and the emissions shift to the more blue region accordingly. Furthermore, to extend the application scope of NHCs as the second cyclometalated ligands, five other Ir(III) complexes from blue to red were synthesized with different first cyclometalated ligands. Finally, the organic light-emitting diodes using one blue emitter exhibit a maximum current efficiency of 37.83 cd A(-1), an external quantum efficiency of 10.3%, and a maximum luminance of 8709 cd m(-2). Our results demonstrate that NHCs as the second cyclometalated ligands are good candidates for the achievement of efficient phosphorescent Ir-III complexes and corresponding devices.DOI:10.1021/ic501949h
-
作为产物:描述:咪唑 、 3-碘三氟甲苯 在 1,10-菲罗啉 、 (dibenzylidene)acetone copper(I) trifluoromethanesulfonate benzene 、 caesium carbonate 作用下, 以 xylene 为溶剂, 反应 24.0h, 以94%的产率得到1-(3-三氟甲基苯基)咪唑参考文献:名称:铜催化芳基卤化物与咪唑的高效偶联摘要:铜催化的咪唑的N-芳基化反应可以使用(CuOTf)2 ·苯作为铜源,而Cs 2 CO 3作为二甲苯中的碱在110–125°C下完成。添加1,10-菲咯啉(phen)和反式,反式-二苄基丙酮(dba)对于该方法的成功至关重要。以高收率分离出产物N-芳基咪唑。DOI:10.1016/s0040-4039(99)00291-9
文献信息
-
Highly efficient and practical phosphoramidite–copper catalysts for amination of aryl iodides and heteroaryl bromides with alkylamines and N(H)-heterocycles作者:Zhanjin Zhang、Jincheng Mao、Di Zhu、Fan Wu、Huilin Chen、Boshun WanDOI:10.1016/j.tet.2006.02.062日期:2006.5A highly efficient copper-catalyzed system using phosphoramidite as ligands was applied to N-arylation of alkylamines and N(H)-heterocycles with aryl iodides and heteroaryl bromides. The reactions were carried out in relative mild conditions and good to excellent yields were obtained.
-
Highly Efficient Copper-Catalyzed Formation of <i>N</i>-Aryl Diazoles Using KF/Al<sub>2</sub>O<sub>3</sub>作者:Rahman Hosseinzadeh、Mahmood Tajbakhsh、Mohammad AlikaramiDOI:10.1055/s-2006-947344日期:2006.8coupling of aryl iodides with heterocyclic compounds such as diazoles that does not require the use of alkoxide bases is described. The C-N bond forming procedure shows that the combination of air stable Cul and 1,10-phenanthroline in the presence of KF/Al 2 O 3 comprises an extremely efficient and general catalyst system for the N-arylation of aryl iodides. Different functionalized aryl iodides were coupled
-
Steric effects on acetate-assisted cyclometallation of <i>meta</i>-substituted <i>N</i>-phenyl and <i>N</i>-benzyl imidazolium salts at [MCl<sub>2</sub>Cp*]<sub>2</sub> (M = Ir, Rh)作者:David L. Davies、Kuldip Singh、Neringa TamosiunaiteDOI:10.1039/d1dt02677a日期:——acetate-assisted cyclometallation to provide mixtures of ortho and para substituted cyclometallated complexes. The effect of the substituents on the isomer ratios is discussed; steric effects are more important in the 6-membered rings derived from the N-benzyl imidazolium salts than 5-membered rings from the N-phenyl salts. Comparisons are made to steric effects with some other common directing groups.
-
[EN] USE OF UREA VARIANTS AS AFFINITY LIGANDS<br/>[FR] UTILISATION DE VARIANTES D'UREES EN TANT QUE LIGANDS A AFFINITE申请人:AMERSHAM BIOSCIENCES AB公开号:WO2004039765A1公开(公告)日:2004-05-13The present invention relates to an IgG-binding compound, which more specifically has affinity for human IgGs of k-type and functional derivatives thereof. More specifically, the compound according to the invention comprises an N,N-alkylated urea moiety located between an aromatic part and another part, which is a linear or cyclic substituted or unsubstituted aliphatic group. The compound binds to a pocket-shaped binding site present on all human IgG k-Fabs, which site is located between the two domains (CH1 and CL) of its constant part. Accordingly, the compound according to the invention is a ligand for human IgGs of k-type, and consequently, the invention also relates to a separation matrix for affinity chromatography, which matrix comprises said compound, as well as to other uses of the compound.
-
METHODS AND COMPOSITIONS FOR TREATING NEURODEGENERATIVE DISEASES申请人:Shakespeare William C.公开号:US20140045826A1公开(公告)日:2014-02-13The invention discloses methods and compositions for treating or preventing neurodegenerative disease by administering a compound of Formula I: or a pharmaceutically acceptable salt, solvate or hydrate thereof, wherein the variables are defined as herein.该发明揭示了通过给予式I化合物或其药学上可接受的盐、溶剂或水合物来治疗或预防神经退行性疾病的方法和组合物,其中变量的定义如本文所述。
表征谱图
-
氢谱1HNMR
-
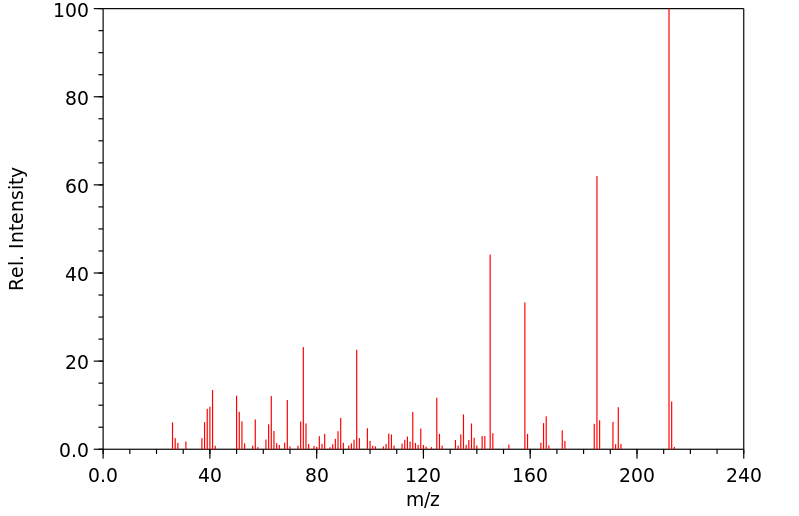
质谱MS
-
碳谱13CNMR
-
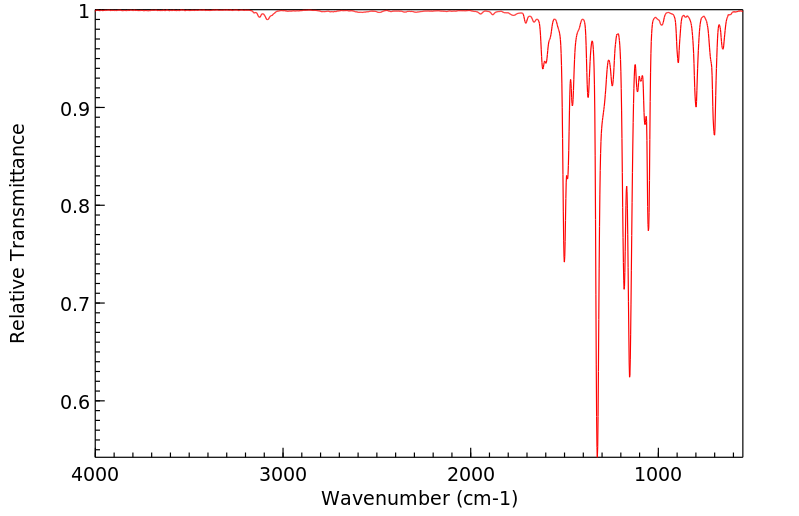
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(SP-4-1)-二氯双(1-苯基-1H-咪唑-κN3)-钯
(5aS,6R,9S,9aR)-5a,6,7,8,9,9a-六氢-6,11,11-三甲基-2-(2,3,4,5,6-五氟苯基)-6,9-甲基-4H-[1,2,4]三唑[3,4-c][1,4]苯并恶嗪四氟硼酸酯
(5-氨基-1,3,4-噻二唑-2-基)甲醇
齐墩果-2,12-二烯[2,3-d]异恶唑-28-酸
黄曲霉毒素H1
高效液相卡套柱
非昔硝唑
非布索坦杂质Z19
非布索坦杂质T
非布索坦杂质K
非布索坦杂质E
非布索坦杂质D
非布索坦杂质67
非布索坦杂质65
非布索坦杂质64
非布索坦杂质61
非布索坦代谢物67M-4
非布索坦代谢物67M-2
非布索坦代谢物 67M-1
非布索坦-D9
非布索坦
非唑拉明
雷非那酮-d7
雷西那德杂质2
雷西纳德杂质L
雷西纳德杂质H
雷西纳德杂质B
雷西纳德
雷西奈德杂质
阿西司特
阿莫奈韦
阿考替胺杂质9
阿米苯唑
阿米特罗13C2,15N2
阿瑞匹坦杂质
阿格列扎
阿扎司特
阿尔吡登
阿塔鲁伦中间体
阿培利司N-1
阿哌沙班杂质26
阿哌沙班杂质15
阿可替尼
阿作莫兰
阿佐塞米
镁(2+)(Z)-4'-羟基-3'-甲氧基肉桂酸酯
锌1,2-二甲基咪唑二氯化物
锌(II)(苯甲醇)(四苯基卟啉)
锌(II)(正丁醇)(四苯基卟啉)
锌(II)(异丁醇)(四苯基卟啉)