杀扑磷 | 950-37-8
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:39-40°C
-
沸点:347.7±52.0 °C(Predicted)
-
密度:1.51 g/cm3
-
闪点:100 °C
-
溶解度:可溶于DMSO(少许)、甲醇(少许)
-
物理描述:Methidathion appears as colorless crystals. This material is used as a non-systemic insecticide. (EPA, 1998)
-
颜色/状态:Colorless crystals
-
气味:Organophosphate odor
-
蒸汽压力:3.37X10-6 mm Hg at 25 °C
-
稳定性/保质期:
在常温常压下,该物质是稳定的。
-
分解:When heated to decomposition it emits very toxic fumes of /oxides of nitrogen, oxides of phosphorous and oxides of sulfur/.
-
腐蚀性:Non-corrosive
-
碰撞截面:160.41 Ų [M+Na]+
-
保留指数:2037;2037;2027;2046;2064;2058;2092;2064;2044.9;2050.2;2063.1;2050.9;2057.5;2046.8;2030;2045;2045.2;2061.5
计算性质
-
辛醇/水分配系数(LogP):2.4
-
重原子数:16
-
可旋转键数:6
-
环数:1.0
-
sp3杂化的碳原子比例:0.666
-
拓扑面积:143
-
氢给体数:0
-
氢受体数:8
ADMET
安全信息
-
危险等级:6.1(a)
-
危险品标志:T+,N
-
安全说明:S22,S28,S36/37,S45,S60,S61
-
危险类别码:R50/53,R28,R21
-
WGK Germany:3
-
RTECS号:TE2100000
-
包装等级:II
-
危险类别:6.1(a)
-
危险品运输编号:UN 2811
-
储存条件:0至6°C下应密封储存。
SDS
| 国标编号: | 61125 |
| CAS: | 950-37-8 |
| 中文名称: | 杀扑磷 |
| 英文名称: | methidathion;Supracide;Vltracide;GS13005;NC2964 |
| 别 名: | 速扑杀;S-2,3-二氢-5-甲氧基-2-氧代-1,3,4-硫二氮茂-3基甲基-O,O-二甲基二硫供磷酸酯 |
| 分子式: | C 4 H 7 Br 2 Cl 2 O 4 P |
| 分子量: | 381 |
| 熔 点: | 34~40℃ |
| 密 度: | 相对密度:1.495(20℃ |
| 蒸汽压: | 0.133mPa (20℃) |
| 溶解性: | 20℃时水中为250ppm,丙酮690g/kg,乙醇260g/kg,环己 |
| 稳定性: | 在中性介质中稳定,在碱中易分解。不易燃、不易爆炸, |
| 外观与性状: | 纯品为无色结晶 |
| 危险标记: | 13(剧毒品) |
| 用 途: | 本品为非内吸性杀虫剂,并具有一定杀螨活性,可防治多种汁食性害虫与叶食性害虫,特别是可防治介壳虫,基本上无药害 |
2.对环境的影响:
杀扑磷对人畜高毒。原药对雄大鼠急性经口毒性LD50为26mg/kg,雌大鼠43.8mg/kg;对大鼠急性经皮毒性LD50为1546mg/kg,兔为200mg/kg。对兔眼睛无刺激作用,对皮肤有轻度刺激。对鱼毒性大,虹鳟LC50(96小时)为0.01mg/L。
杀扑磷为有机磷杀虫剂对害虫和螨类有触杀和胃毒作用。
3.现场应急监测方法:
直接进水样气相色谱法
4.实验室监测方法:
气相色谱法(GB/T14552-93,水和土壤)
5.环境标准:
前西德(1978)食物中最高残留限值: 0.2~15mg/kg(特定农作物);0.1mg/kg(一般农作物)
6.应急处理处置方法:
包装:可采用壁厚不小于1.2mm的铁桶,桶口严封不漏,每桶净重不应超过200kg,或以玻瓶或塑料瓶装后外包木箱,每箱净重不超过25kg。箱或桶外要贴上“剧毒品”标志。
消防:可用水、砂土、二氧化碳灭火。在灭火时应穿戴防护用品以防中毒。
急救:本品为高毒类有机磷农药,是强力羧基酯酶抑制剂。急性中毒,若皮肤污染应迅速脱去污染处衣物,用3~5%碳酸氢钠冲洗。溅入眼内,用2%碳酸氢钠或生理盐水冲洗10分钟以上,再滴1~2滴1%阿托品。口服中毒应尽早探咽导吐并洗胃,(可选2%碳酸氢钠或淡食盐水),必要时肌肉注射可托品。
贮存与运输:本品应存放在干燥、阴凉的库房内,避免阳光照射,不可与氧化剂、易燃品与食用化工产品共存混运。搬运时应小心,避免损坏包装造成产品泄漏。工作人员应穿戴可靠的劳保用品。
制备方法与用途
杀扑磷属于二硫代磷酸酯类杀虫剂,是一种广谱有机磷杀虫剂。它对害虫和螨类具有触杀和胃毒作用,尤其在防治蚧类方面效果显著,并可防治多种棉花害虫。
作用机制杀扑磷具有胃毒及触杀作用,能够渗透植物组织,即使风雨淋洗也不怕,可以杀死叶片或果皮中的害虫,也能杀死叶背未接触农药的害虫。
残效期及药害在苹果上施用稀释1000倍的杀扑磷20天后,仍能对苹果考蛾幼虫保持95%的有效防治率。对柑桔褐园螨施用该药液1000倍稀释30天以上仍有显著效果。在使用40%环乳油稀释400倍喷洒苹果树嫩叶时,连续观察10天无明显药害。
毒性雄性和雌性大鼠的急性经口LD₅₀分别为43.8 mg/kg和26 mg/kg。皮肤接触LD₅₀为150 mg/kg(皮下1546 mg/kg)。兔子的急性经皮LD₅₀为200 mg/kg,对眼睛无刺激作用,但对皮肤有轻微刺激性。大鼠两年饲喂试验的最大无作用剂量为每天0.15 mg/kg 或者 0.25 mg/kg。动物实验未发现杀扑磷具有致畸、致癌或致突变的作用,并且在三代繁殖试验和神经毒性测试中也未见异常现象。虹鳟鱼的LC₅₀值为0.01 mg/L(96小时)。
化学性质纯品为无色结晶,熔点39~40℃ (1.33Pa),相对密度1.495(20℃)。在常温下可稳定储存2年,在弱酸性和中性介质中稳定,遇碱性条件易水解。杀扑磷不易燃、不易爆。在不同溶剂中的溶解度如下:环己酮850 g/kg,丙酮690 g/kg,二甲苯600 g/kg,乙醇260 g/kg,水中为250 mg/kg。
用途杀扑磷具有触杀和胃毒作用;它渗透性强,但无内吸作用。是一种高毒、广谱的杀虫剂兼杀螨剂,特别适用于防治介壳虫。用于防治棉蚜、棉叶蝉、棉盲蝽等,每100㎡喷洒40%乳油7.5~11.3 mL对水喷雾;防治柑橘矢尖蚧、糠片蚧、蜡蚧(男性),使用该药液1000倍稀释即可。杀扑磷也可用于预防和控制各种棉花害虫。
合成工艺杀扑磷的合成包括三个步骤:
- 羰基肼的制备:在反应锅中加入水并添加羰基肼,在60℃下滴加氯甲酸三氯甲酯,保温2小时后离心,得到淡黄色噻二唑酮晶体。
- 噻二唑酮与甲醛及硫酸的合成:加入水和噻二唑酮,并在55-60℃下控制温度滴加氯甲酸三氯甲酯,保温后再进行分离、洗涤,制备出白色杀扑磷原药。
- 羰基肼与甲基硫代物的反应:75%硫酸中加入噻二唑酮和溶剂A以及催化剂后升温至20℃并滴加甲醛,随后降温至40℃保温2小时。静置分层后,加入水洗涤两次,在低温下结晶得到白色杀扑磷原药,含量为96%-98%,收率42%。
文献报道汽巴-嘉基公司和以色列亚姆公司的合成工艺采用光气法制造噻二唑酮,并将该化合物与O,O-二甲基二硫代磷酸反应制备得到。
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 3-(二甲氧基磷酰巯基甲基)-5-甲氧基-1,3,4-噻二唑-2-酮 thiophosphoric acid S-(5-methoxy-2-oxo-[1,3,4]thiadiazol-3-ylmethyl) ester O,O'-dimethyl ester 39856-16-1 C6H11N2O5PS2 286.269 —— 2-Methoxy-4-mercaptomethyl-5-oxo-Δ2-1,3,4-thiadiazolin 58458-70-1 C4H6N2O2S2 178.236
反应信息
-
作为反应物:描述:杀扑磷 在 sodium hypochlorite 作用下, 以 aq. phosphate buffer 为溶剂, 反应 168.0h, 生成 3-(二甲氧基磷酰巯基甲基)-5-甲氧基-1,3,4-噻二唑-2-酮参考文献:名称:氯化对有机磷杀虫剂溶液的抗乙酰胆碱酯酶活性的影响以及母体杀虫剂及其氧酮对活性的贡献。摘要:已知有机磷杀虫剂在饮用水处理的氯化步骤中会部分转化为它们各自的氧酮。对于大多数有机磷杀虫剂而言,确定可接受的每日摄入量的毒理学终点是抑制乙酰胆碱酯酶(AChE)。像母体杀虫剂一样,oxon也会抑制AChE,因此也要评估饮用水中oxox的存在。但是,除氧子以外,没有注意可能存在的转化产物(TPs)。在本研究中,我们确定观察到的有机磷杀虫剂马拉硫磷和甲硫磷的氯化溶液的抗AChE活性是否可以仅归因于母体化合物及其氧酮。氯化后 马拉硫磷和甲硫磷都立即转化为它们的牛。最大转化率分别为60%和30%,表明这些化合物中至少有40%和70%被转化为其他TP。氯化前,含马拉硫磷和甲硫磷的溶液几乎没有抗AChE活性,但是氯化后溶液显示出很强的活性。根据样品中化合物的浓度和化合物的化学标准品的剂量反应曲线,可以计算出母体杀虫剂及其含氧化合物对氯化物活性的贡献。对于含马拉硫磷的溶液和含甲硫磷的溶液,计算得出的抗ADOI:10.1016/j.chemosphere.2020.127743
-
作为产物:描述:参考文献:名称:ArbeitenüberPhosphorsäure-andThiophosphorsäureestermit einem heterocyclischen Subitententen。8. Mitteilung。达斯史陶比尔-Verfahren,EINE Eintopf-Kondensation冯Thiophosphorverbindungen(,XO,S),Aldehyden UND Heterocyclen(MIT苏拉NH-GRUPPE)†摘要:硫代化合物的缩合反应1,醛和杂环3用酸性NH基团在特定浓度的强无机酸,以非对称的化合物4,称为史陶比尔从过程,不同的曼尼希型反应通过弱或稀酸(氨基烷基化的催化(Hellmann&Opitz)和Tscherniac-Einhorn型在浓硫酸中的缩合反应(Hellmann的酰胺甲基化)。它的主要特征是两个缩合反应参与者1和3的酸度; 因此,需要将无机酸的浓度调节到各自最佳的程度。然而,它们的p K差异足以区分它们的亲核性和反应性,并防止它们与乙醛的副反应同时发生,从而反应成对称副产物(如13、14和15)。DOI:10.1002/hlca.19740570619
-
作为试剂:描述:碘甲基环戊烷 、 Ethyl 2-(4-piperazin-1-ylsulfonylphenyl)acetate 在 lithium diisopropyl amide 作用下, 以 四氢呋喃 、 正己烷 、 杀扑磷 为溶剂, 反应 15.25h, 生成 Ethyl 3-cyclopentyl-2-(4-piperazin-1-ylsulfonylphenyl)propanoate参考文献:名称:Investigation of Functionally Liver Selective Glucokinase Activators for the Treatment of Type 2 Diabetes摘要:Type 2 diabetes is a polygenic disease which afflicts nearly 200 million people worldwide and is expected to increase to near epidemic levels over the next 10-15 years. Glucokinase (GK) activators are currently under investigation by a number of pharmaceutical companies with only a few reaching early clinical evaluation. A GK activator has the promise of potentially affecting both the beta-cells of the pancreas, by improving glucose sensitive insulin secretion, as well as the liver, by reducing uncontrolled glucose output and restoring post-prandial glucose uptake and storage as glycogen. Herein, we report our efforts on a sulfonamide chemotype with the aim to generate liver selective GK activators which culminated in the discovery of 3-cyclopentyl-N-(5-methoxy-thiazolo[5,4-b]pyridin-2-yl)-2-[4-(4-methylpiperazine-1-sulfonyl)-phenyl]-propionamide (17c). This compound activated the GK enzyme (alpha K(a) = 39 nM) in vitro at low nanomolar concentrations and significantly reduced glucose levels during an oral glucose tolerance test in normal mice.DOI:10.1021/jm900839k
文献信息
-
[EN] ACC INHIBITORS AND USES THEREOF<br/>[FR] INHIBITEURS DE L'ACC ET UTILISATIONS ASSOCIÉES
-
[EN] BICYCLYL-SUBSTITUTED ISOTHIAZOLINE COMPOUNDS<br/>[FR] COMPOSÉS ISOTHIAZOLINE SUBSTITUÉS PAR UN BICYCLYLE申请人:BASF SE公开号:WO2014206910A1公开(公告)日:2014-12-31The present invention relates to bicyclyl-substituted isothiazoline compounds of formula (I) wherein the variables are as defined in the claims and description. The compounds are useful for combating or controlling invertebrate pests, in particular arthropod pests and nematodes. The invention also relates to a method for controlling invertebrate pests by using these compounds and to plant propagation material and to an agricultural and a veterinary composition comprising said compounds.本发明涉及公式(I)中变量如索权和说明中所定义的自行车基取代异噻唑啉化合物。这些化合物对抗或控制无脊椎动物害虫,特别是节肢动物害虫和线虫方面具有用途。该发明还涉及一种通过使用这些化合物来控制无脊椎动物害虫的方法,以及包含所述化合物的植物繁殖材料、农业和兽医组合物。
-
[EN] AZOLINE COMPOUNDS<br/>[FR] COMPOSÉS AZOLINE申请人:BASF SE公开号:WO2015128358A1公开(公告)日:2015-09-03The present invention relates to azoline compounds of formula (I) wherein A, B1, B2, B3, G1, G2, X1, R1, R3a, R3b, Rg1 and Rg2 are as defined in the claims and the description. The compounds are useful for combating or controlling invertebrate pests, in particular arthropod pests and nematodes. The invention also relates to a method for controlling invertebrate pests by using these compounds and to plant propagation material and to an agricultural and a veterinary composition comprising said compounds.本发明涉及式(I)的噁唑啉化合物,其中A、B1、B2、B3、G1、G2、X1、R1、R3a、R3b、Rg1和Rg2如权利要求和描述中所定义。这些化合物对抗或控制无脊椎动物害虫,特别是节肢动物害虫和线虫方面具有用途。该发明还涉及一种利用这些化合物控制无脊椎动物害虫的方法,以及包括所述化合物的植物繁殖材料、农业和兽医组合物。
-
[EN] MICROBIOCIDAL OXADIAZOLE DERIVATIVES<br/>[FR] DÉRIVÉS D'OXADIAZOLE MICROBIOCIDES申请人:SYNGENTA PARTICIPATIONS AG公开号:WO2017157962A1公开(公告)日:2017-09-21Compounds of the formula (I) wherein the substituents are as defined in claim 1, useful as a pesticides, especially fungicides.式(I)的化合物,其中取代基如权利要求1所定义,作为杀虫剂特别是杀菌剂有用。
-
Thieno-pyrimidine compounds having fungicidal activity
表征谱图
-
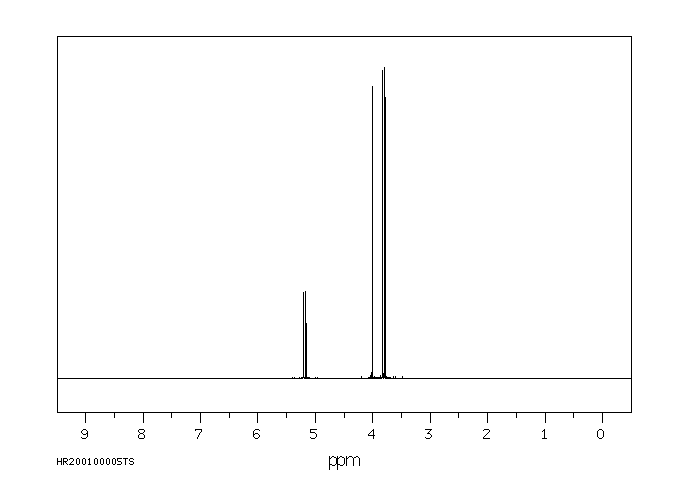
氢谱1HNMR
-
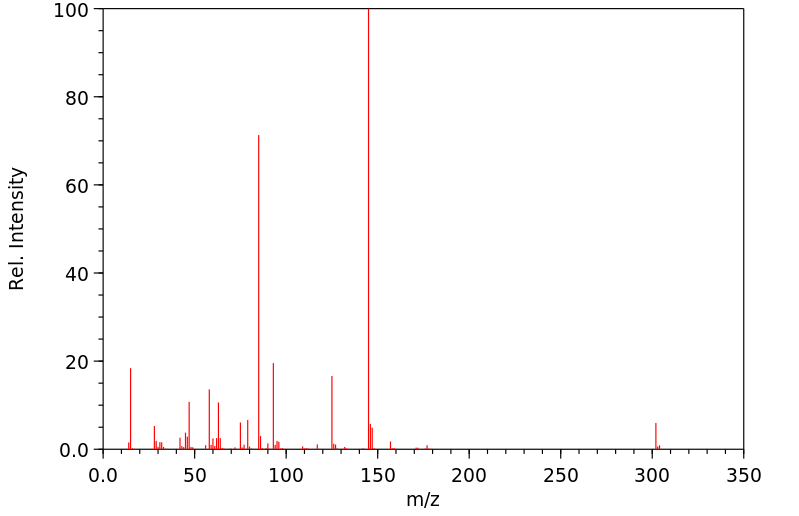
质谱MS
-
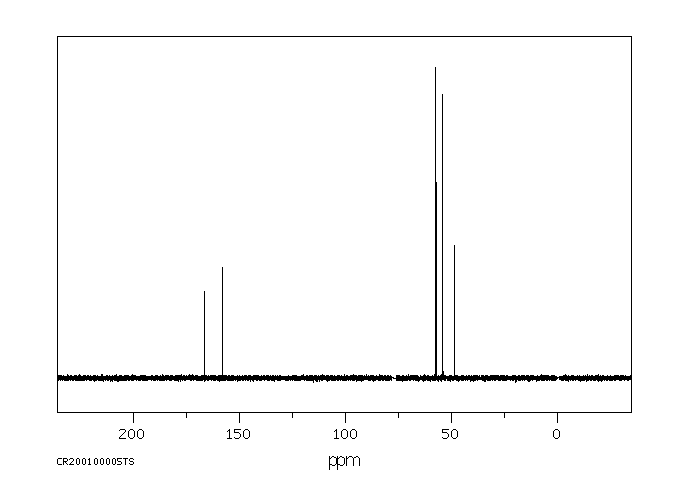
碳谱13CNMR
-
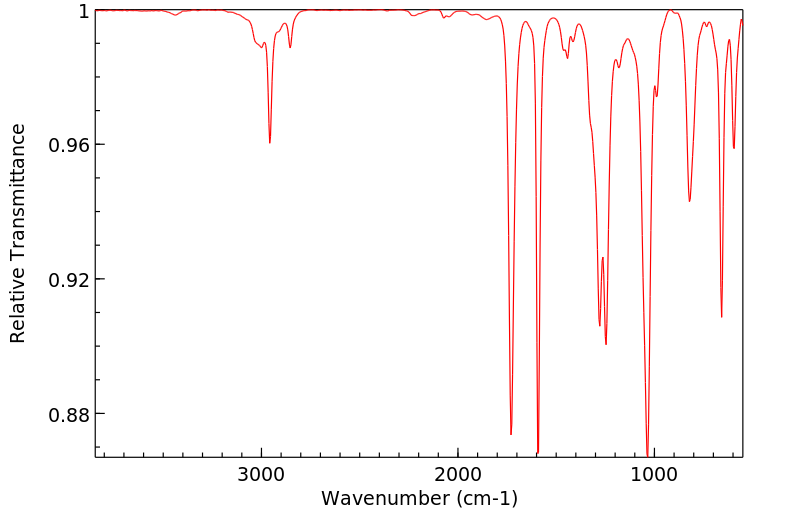
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息