1-乙基溴化吡啶 | 1906-79-2
中文名称
1-乙基溴化吡啶
中文别名
1-乙基吡啶氢溴酸盐;N-乙基吡啶溴盐;乙基溴化吡啶;溴化N-乙基吡啶
英文名称
1-ethylpyridinium bromide
英文别名
N-ethylpyridinium bromide;1-ethylpyridin-1-ium bromide;ethylpyridinium bromide;1-ethylpyridin-1-ium;bromide
CAS
1906-79-2
化学式
Br*C7H10N
mdl
——
分子量
188.067
InChiKey
ABFDKXBSQCTIKH-UHFFFAOYSA-M
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:117-121 °C
-
密度:1.4194 (rough estimate)
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):-2.0
-
重原子数:9
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.29
-
拓扑面积:3.9
-
氢给体数:0
-
氢受体数:1
安全信息
-
TSCA:Yes
-
危险品标志:Xi
-
安全说明:S26,S37/39
-
危险类别码:R36/37/38
-
海关编码:2933399090
-
储存条件:请将贮藏器保持密封状态,并存放在阴凉、干燥处。同时,确保工作环境具有良好的通风或排气设施。
SDS
1-乙基溴化吡啶鎓 修改号码:5
模块 1. 化学品
产品名称: 1-Ethylpyridinium Bromide
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 1-乙基溴化吡啶鎓
百分比: >98.0%(LC)(T)
CAS编码: 1906-79-2
分子式: C7H10BrN
1-乙基溴化吡啶鎓 修改号码:5
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
存放于惰性气体环境中。
防湿。
远离不相容的材料比如氧化剂存放。
易湿
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
1-乙基溴化吡啶鎓 修改号码:5
模块 9. 理化特性
外观: 晶体-粉末
颜色: 白色类白色
气味: 无资料
pH: 无数据资料
熔点:
120°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx), 溴化氢
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
1-乙基溴化吡啶鎓 修改号码:5
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: 1-Ethylpyridinium Bromide
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 1-乙基溴化吡啶鎓
百分比: >98.0%(LC)(T)
CAS编码: 1906-79-2
分子式: C7H10BrN
1-乙基溴化吡啶鎓 修改号码:5
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
存放于惰性气体环境中。
防湿。
远离不相容的材料比如氧化剂存放。
易湿
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
1-乙基溴化吡啶鎓 修改号码:5
模块 9. 理化特性
外观: 晶体-粉末
颜色: 白色类白色
气味: 无资料
pH: 无数据资料
熔点:
120°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx), 溴化氢
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
1-乙基溴化吡啶鎓 修改号码:5
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
反应信息
-
作为反应物:描述:参考文献:名称:Horner; Schwenk, Justus Liebigs Annalen der Chemie, 1950, vol. 566, p. 69,82摘要:DOI:
-
作为产物:参考文献:名称:Dual functional ionic liquids as plasticisers and antimicrobial agents for medical polymers摘要:耐甲氧西林金黄色葡萄球菌(MRSA)等细菌对医疗器械的污染是临床上非常关注的问题。聚氯乙烯被广泛用于生产导管等医疗器械。导管管材的柔韧性来自于增塑剂的添加。在此,我们报告了两种双功能离子液体(1-乙基吡啶鎓多聚物酸酯和三丁基(2-羟乙基)鏻多聚物酸酯)的设计,它们不仅具有独特的增塑作用,而且对一系列抗生素耐药菌具有抗菌和抗生物膜形成活性。聚氯乙烯的塑化是离子液体浓度的函数。这两种离子液体的有效抗菌性能源于阴离子或阳离子的化学结构,并不局限于阴离子/阳离子上烷基链的长度。所采用的设计方法将有助于开发作为聚合物多功能添加剂的离子液体。DOI:10.1039/c1gc15132k
-
作为试剂:参考文献:名称:含1-乙基溴化吡啶鎓的水电解质中的锌电沉积:意外的异味*摘要:通过循环伏安法,计时电流法和扫描电子显微镜研究了在含溴化锌(50 mM)和1-乙基溴化吡啶鎓([C 2 Py] Br,50 mM)的水性电解质中锌的可逆电沉积。在[C 2]存在下观察到Zn / Zn II氧化还原对的伏安行为异常Py] Br。在一个单一的沉积-剥离循环之后,在相对于Ag / AgCl的开关电位比-1.25 V负的开关电位下,观察到了氧化还原对的钝化。这种不寻常的行为归因于1-乙基吡啶鎓阳离子还原为吡啶基自由基及其后续反应,从而影响了锌的电化学。进一步观察到这种行为改变了电沉积的成核过程,从而改变了锌电沉积的形态。DOI:10.1071/ch17281
文献信息
-
A Convenient Synthesis of Triflate Anion Ionic Liquids and Their Properties作者:Nikolai V. Ignat’ev、Peter Barthen、Andryi Kucheryna、Helge Willner、Peter SartoriDOI:10.3390/molecules17055319日期:——synthesis of high purity triflate ionic liquids via direct alkylation of organic bases (amines, phosphines or heterocyclic compounds) with methyl and ethyl trifluoromethanesulfonate (methyl and ethyl triflate) has been developed. Cheap and non-toxic dimethyl and diethyl carbonate serve as source for the methyl and ethyl groups in the preparation of methyl and ethyl triflate by this invented process. The properties
-
Reaction Mechanism of Cathodic Crossed Coupling of Acetone with Unsaturated Compounds in Acidic Solution作者:Toshio Koizumi、Toshio Fuchigami、Zaghloul El-Shahat Kandeel、Norio Sato、Tsutomu NonakaDOI:10.1246/bcsj.59.757日期:1986.3confirmed that the cathodic crossed coupling of acetone with unsaturated compounds in aqueous sulfuric acid could proceed smoothly, when the compounds which had radical-acceptable double bonds and were adsorbed on a mercury cathode were used. From this fact, it was concluded that the coupling occurs via the addition of a radical intermediate formed by the one-electron reduction of acetone to the double
-
PROCESS FOR PRODUCING OPTICALLY ACTIVE ALCOHOL申请人:Hatakeyama Taito公开号:US20110282077A1公开(公告)日:2011-11-17The object of the present invention is to solve the problems in the prior arts, and to find more improved reaction conditions for suppressing the racemization of the product and obtaining an optically active alcohol at a high optical purity. The inventors achieved to solve the above problems by using a solvent system that is capable of resolving both an asymmetric catalyst and a formate salt, allowing the hydrogen source and the asymmetric catalyst to be present in the same phase.本发明的目的是解决先前技术中的问题,并找到更改进的反应条件,以抑制产物的消旋化并获得高光学纯度的光学活性醇。发明者通过使用一种能够分离不对称催化剂和甲酸盐的溶剂系统,使氢源和不对称催化剂能够存在于同一相中,从而解决了上述问题。
-
Boosting activity of molecular oxygen by pyridinium-based photocatalysts for metal-free alcohol oxidation作者:Shuai Ma、Jing-Wang Cui、Cai-Hui Rao、Meng-Ze Jia、Yun-Rui Chen、Jie ZhangDOI:10.1039/d0gc03730c日期:——oxygen species can be achieved, and a photoinduced electron transfer catalytic system for the oxidation of alcohols has been developed. Thus, we have successfully simplified the complicated catalytic system into a single molecular catalyst without any additional noble metals and co-catalysts/additives. The current photocatalytic system shows high catalytic efficiency not only for aromatic alcohols but
-
Does alkyl chain length really matter? Structure–property relationships in thermochemistry of ionic liquids作者:Sergey P. Verevkin、Dzmitry H. Zaitsau、Vladimir N. Emel’yanenko、Ricardas V. Ralys、Andrei V. Yermalayeu、Christoph SchickDOI:10.1016/j.tca.2013.04.003日期:2013.6Abstract DSC was used for determination of reaction enthalpies of synthesis of ionic liquids [Cnmim][Cl]. A combination of DSC with quantum chemical calculations presents an indirect way to study thermodynamics of ionic liquids. The indirect procedure for vaporization enthalpy was validated with the direct experimental measurements by using thermogravimetry. First-principles calculations of the enthalpy
表征谱图
-
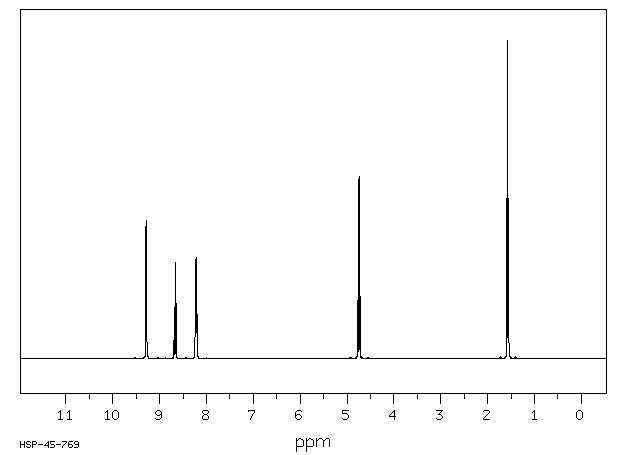
氢谱1HNMR
-
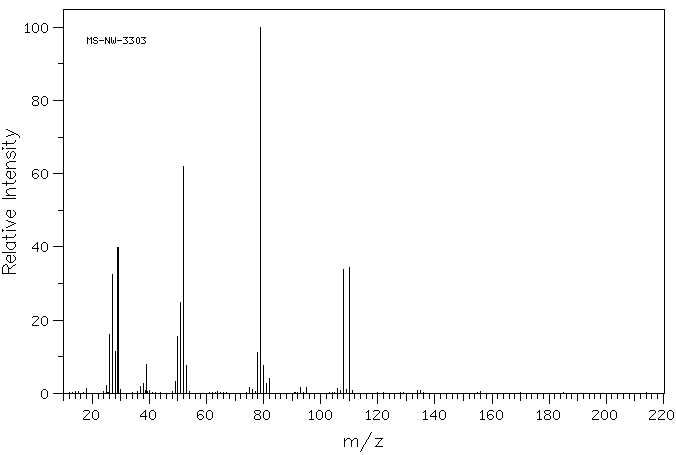
质谱MS
-
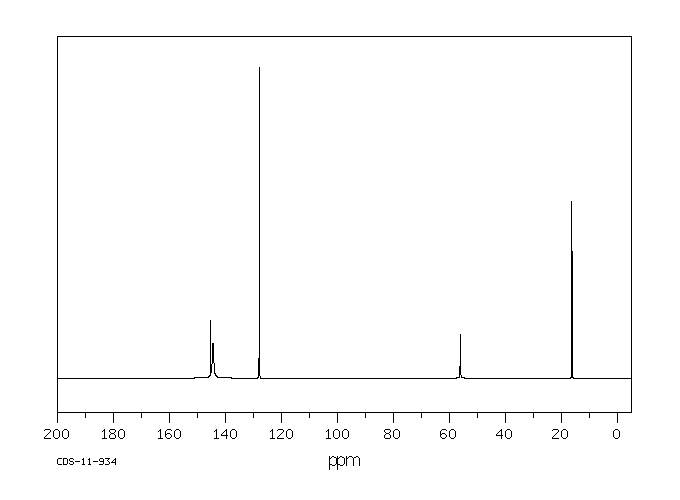
碳谱13CNMR
-
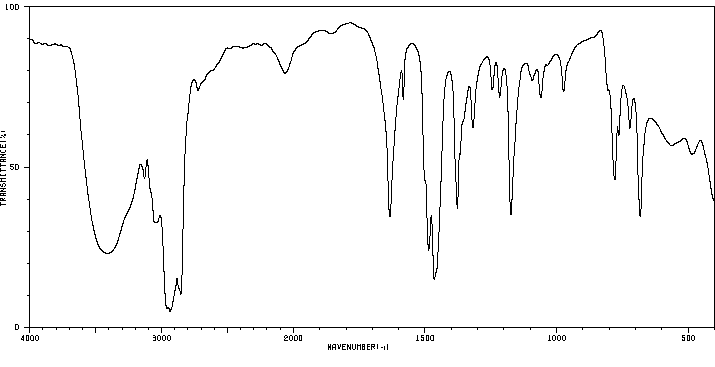
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-氨氯地平-d4
(R,S)-可替宁N-氧化物-甲基-d3
(R)-(+)-2,2'',6,6''-四甲氧基-4,4''-双(二苯基膦基)-3,3''-联吡啶(1,5-环辛二烯)铑(I)四氟硼酸盐
(R)-N'-亚硝基尼古丁
(R)-DRF053二盐酸盐
(5E)-5-[(2,5-二甲基-1-吡啶-3-基-吡咯-3-基)亚甲基]-2-亚磺酰基-1,3-噻唑烷-4-酮
(5-溴-3-吡啶基)[4-(1-吡咯烷基)-1-哌啶基]甲酮
(5-氨基-6-氰基-7-甲基[1,2]噻唑并[4,5-b]吡啶-3-甲酰胺)
(2S,2'S)-(-)-[N,N'-双(2-吡啶基甲基]-2,2'-联吡咯烷双(乙腈)铁(II)六氟锑酸盐
(2S)-2-[[[9-丙-2-基-6-[(4-吡啶-2-基苯基)甲基氨基]嘌呤-2-基]氨基]丁-1-醇
(2R,2''R)-(+)-[N,N''-双(2-吡啶基甲基)]-2,2''-联吡咯烷四盐酸盐
(1'R,2'S)-尼古丁1,1'-Di-N-氧化物
黄色素-37
麦斯明-D4
麦司明
麝香吡啶
鲁非罗尼
鲁卡他胺
高氯酸N-甲基甲基吡啶正离子
高氯酸,吡啶
高奎宁酸
马来酸溴苯那敏
马来酸氯苯那敏-D6
马来酸左氨氯地平
顺式-双(异硫氰基)(2,2'-联吡啶基-4,4'-二羧基)(4,4'-二-壬基-2'-联吡啶基)钌(II)
顺式-二氯二(4-氯吡啶)铂
顺式-二(2,2'-联吡啶)二氯铬氯化物
顺式-1-(4-甲氧基苄基)-3-羟基-5-(3-吡啶)-2-吡咯烷酮
顺-双(2,2-二吡啶)二氯化钌(II) 水合物
顺-双(2,2'-二吡啶基)二氯化钌(II)二水合物
顺-二氯二(吡啶)铂(II)
顺-二(2,2'-联吡啶)二氯化钌(II)二水合物
韦德伊斯试剂
非那吡啶
非洛地平杂质C
非洛地平
非戈替尼
非布索坦杂质66
非尼拉朵
非尼拉敏
雷索替丁
阿雷地平
阿瑞洛莫
阿扎那韦中间体
阿培利司N-6
阿伐曲波帕杂质40
间硝苯地平
间-硝苯地平
镉,二碘四(4-甲基吡啶)-
锌,二溴二[4-吡啶羧硫代酸(2-吡啶基亚甲基)酰肼]-