1-环己基-2-丁烯醇 | 79646-42-7
中文名称
1-环己基-2-丁烯醇
中文别名
(1-羟基-2-丁烯基)环己烷
英文名称
(E)-1-cyclohexyl-2-buten-1-ol
英文别名
(±)-(E)-1-cyclohexylbut-2-en-1-ol;(E)-1-cyclohexylbut-2-en-1-ol;1-cyclohexyl-2-buten-1-ol;1-cyclohexylbut-2-en-1-ol;(+/-)-1-cyclohexyl-but-2t-en-1-ol
CAS
79646-42-7;106499-63-2;120849-47-0;130930-52-8;18736-82-8;137764-95-5;79605-62-2
化学式
C10H18O
mdl
——
分子量
154.252
InChiKey
WZIIBXRIIUAFOT-QHHAFSJGSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:114 °C / 18mmHg
-
密度:0.93
-
闪点:66 °C
-
稳定性/保质期:
在常温常压下稳定,应避免接触氧化物。
计算性质
-
辛醇/水分配系数(LogP):2.8
-
重原子数:11
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.8
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
安全信息
-
储存条件:常温下应密闭避光保存,并放置于通风干燥处。
反应信息
-
作为反应物:描述:参考文献:名称:Cobalt(II)-Catalyzed Conversion of Allylic Alcohols/Acetates to Allylic Amides in the Presence of Nitriles摘要:Various secondary allylic alcohols or their acetates and tertiary allylic alcohols can be converted to the corresponding transposed allylic amides in the presence of a catalytic quantity of cobalt(II) chloride and acetic anhydride in acetonitrile. Tertiary alcohols undergo complete rearrangement whereas secondary ones afford a mixture of regioisomers. Moderate yields of amides are also obtained by reacting acrylonitrile with secondary alcohols in 1,2-dichloroethane. The presence of acetic anhydride or acetic acid is crucial to the formation of amides as the absence of the former affords no amides and the allylic alcohols are mainly recovered as regioisomeric mixtures. The regioselectivity during amide formation can be enhanced by using cobalt complexes 14-16 in acetic acid medium. Some preliminary studies indicate that these reactions are proceeding via an pi-allyl complex or tight ion pair rather than a [3,3] sigmatropic rearrangement of acetamidate obtained in a Pinner reaction.DOI:10.1021/jo00114a013
-
作为产物:参考文献:名称:Concomitant Epoxide Deoxygenation and Deacetylation of Glycidyl Acetates Induced by Telluride Ion摘要:Treatment of glycidyl acetates with telluride ion (Te2-) produced by reduction of elemental Te with LiEt(3)BH yields allylic alcohols by loss of the epoxide oxygen atom and the acetyl group from the ester. If the glycidyl acetate is disubstituted at C-3, a rearrangement to an isomeric allylic alcohol competes with the deoxygenation-deacetylation. Triethylborane, a byproduct in the reduction of Te, is believed to play an important role as a Lewis acid since when it is absent or removed by addition of fluoride ion the reaction is extremely slow.DOI:10.1021/jo00084a016
文献信息
-
One-Pot Synthesis of Optically Active Allyl Esters via Lipase−Vanadium Combo Catalysis作者:Shuji Akai、Ryosuke Hanada、Noboru Fujiwara、Yasuyuki Kita、Masahiro EgiDOI:10.1021/ol102053a日期:2010.11.5with a lipase produced the regio- and enantioconvergent transformation of racemic allyl alcohols (1 or 2) into optically active allyl esters. In this system, the vanadium compounds catalyzed the continuous racemization of the alcohols along with the transposition of the hydroxyl group, while the lipase effected the chemo- and enantioselective esterification to achieve the dynamic kinetic resolution.
-
A Mesoporous-Silica-Immobilized Oxovanadium Cocatalyst for the Lipase-Catalyzed Dynamic Kinetic Resolution of Racemic Alcohols作者:Masahiro Egi、Koji Sugiyama、Moriaki Saneto、Ryosuke Hanada、Katsuya Kato、Shuji AkaiDOI:10.1002/anie.201208988日期:2013.3.25V for resolution: A new oxovanadium catalyst (V‐MPS; see scheme) immobilized in the pores of mesoporous silica has been developed. The combined use of V‐MPS and lipases achieved the dynamic kinetic resolution of a wide range of racemic alcohols (1 or 2) to produce optically active esters 3 in high chemical and optical yields. The paired catalysts retained high catalytic activity when reused up to six
-
Gold(I)-Catalysed Direct Thioetherifications Using Allylic Alcohols: an Experimental and Computational Study作者:Lorena Herkert、Samantha L. J. Green、Graeme Barker、David G. Johnson、Paul C. Young、Stuart A. Macgregor、Ai-Lan LeeDOI:10.1002/chem.201403293日期:2014.9.1A gold(I)‐catalysed direct thioetherification reaction between allylic alcohols and thiols is presented. The reaction is generally highly regioselective (SN2′). This dehydrative allylation procedure is very mild and atom economical, producing only water as the by‐product and avoiding any unnecessary waste/steps associated with installing a leaving or activating group on the substrate. Computational
-
Highly Stereoselective C–C Bond Formation by Rhodium-Catalyzed Tandem Ylide Formation/[2,3]-Sigmatropic Rearrangement between Donor/Acceptor Carbenoids and Chiral Allylic Alcohols作者:Zhanjie Li、Brendan T. Parr、Huw M. L. DaviesDOI:10.1021/ja303023n日期:2012.7.4The tandem ylide formation/[2,3]-sigmatropic rearrangement between donor/acceptor rhodium carbenoids and chiral allyl alcohols is a convergent C-C bond forming process, which generates two vicinal stereogenic centers. Any of the four possible stereoisomers can be selectively synthesized by appropriate combination of the chiral catalyst Rh(2)(DOSP)(4) and the chiral alcohol.
-
Iron-Catalyzed Aerobic Oxidation of Allylic Alcohols: The Issue of C═C Bond Isomerization作者:Jinxian Liu、Shengming MaDOI:10.1021/ol402434x日期:2013.10.18An aerobic oxidation of allylic alcohols using Fe(NO3)3·9H2O/TEMPO/NaCl as catalysts under atmospheric pressure of oxygen at room temperature was developed. This eco-friendly and mild protocol provides a convenient pathway to the synthesis of stereodefined α,β-unsaturated enals or enones with the retention of the C–C double-bond configuration.
表征谱图
-
氢谱1HNMR
-
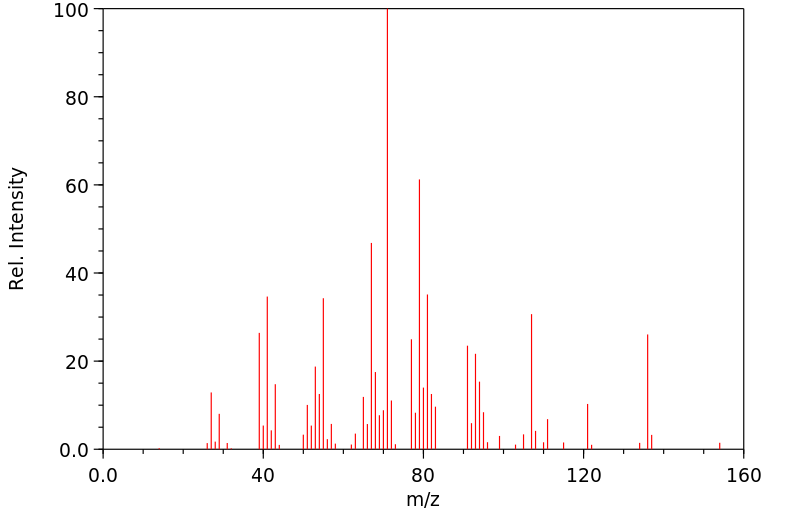
质谱MS
-
碳谱13CNMR
-
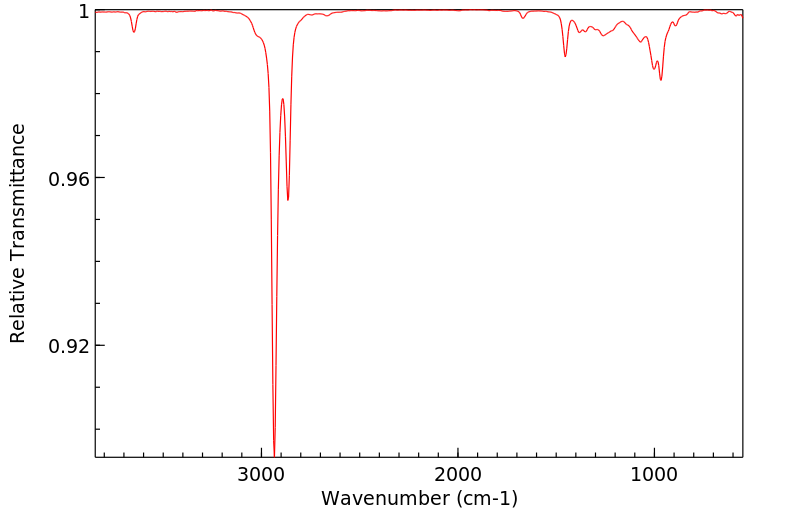
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷