1-苯并噻吩-3-羧酸 | 5381-25-9
中文名称
1-苯并噻吩-3-羧酸
中文别名
苯并[b]噻吩-3-羧酸;苯并[b]噻吩-3-甲酸;苯并[B]噻吩-3-羧酸
英文名称
benzo[b]thiophene-3-carboxylic acid
英文别名
benzothiophene-3-carboxylic acid;1-benzothiophene-3-carboxylic acid;Benzothiophen-3-carbonsaeure;benzothiophene-3-carboxylic acid;3-benzothiophene carboxylic acid;3-Benzothiophencarbonsaeure;Thionaphthen-carbonsaeure-(3)
CAS
5381-25-9
化学式
C9H6O2S
mdl
MFCD01846406
分子量
178.211
InChiKey
DRBLTQNCQJXSNU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:174 °C
-
沸点:305-309 °C
-
密度:1.419±0.06 g/cm3(Predicted)
-
稳定性/保质期:
在常温常压下保持稳定
计算性质
-
辛醇/水分配系数(LogP):2.6
-
重原子数:12
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:65.5
-
氢给体数:1
-
氢受体数:3
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
安全说明:S26,S37/39
-
危险类别码:R36/37/38
-
海关编码:2934999090
-
危险性防范说明:P261,P280,P305+P351+P338
-
危险性描述:H302,H315,H319,H332,H335
-
储存条件:请将药品存放在避光、阴凉干燥处,并密封保存。
SDS
| Name: | 1-Benzothiophene-3-carboxylic acid 97% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 5381-25-9 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 5381-25-9 | 1-Benzothiophene-3-carboxylic acid | 97% | unlisted |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.
Potential Health Effects
Eye:
Causes eye irritation.
Skin:
Causes skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. May be harmful if swallowed.
Inhalation:
Causes respiratory tract irritation. May be harmful if inhaled.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 5381-25-9: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: white
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 175 - 176 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C9H6O2S
Molecular Weight: 178
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Strong oxidizing agents, bases.
Hazardous Decomposition Products:
Carbon monoxide, oxides of sulfur, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 5381-25-9 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
1-Benzothiophene-3-carboxylic acid - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
No information available.
IMO
No information available.
RID/ADR
No information available.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 37/39 Wear suitable gloves and eye/face
protection.
WGK (Water Danger/Protection)
CAS# 5381-25-9: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 5381-25-9 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 5381-25-9 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 苯并噻吩-3-羧酸甲酯 methyl benzothiophene-3-carboxylate 22913-25-3 C10H8O2S 192.238 3-甲醛苯并噻吩 benzothiophene-3-carboxaldehyde 5381-20-4 C9H6OS 162.212 3-乙酰硫茚 3-acetylbenzothiophene 1128-05-8 C10H8OS 176.239 —— benzo[b]thiophene-2,3-dicarboxylic acid 37412-26-3 C10H6O4S 222.221 3-甲基苯噻吩 3-methylbenzothiophene 1455-18-1 C9H8S 148.229 3-丙酰基苯并(b)噻吩 1-benzo[b]thiophen-3-yl-propan-1-one 19492-96-7 C11H10OS 190.266 —— 3-methoxycarbonylbenzothiophene-2-carboxylic acid 87807-50-9 C11H8O4S 236.248 —— dimethyl benzo[b]thiophene-2,3-dicarboxylate 41892-84-6 C12H10O4S 250.275 苯并[B]噻吩-3-甲醛 benzo[b]thiophene-3-carbonitrile 24434-84-2 C9H5NS 159.211 2-氯-3-甲基苯并(b)噻吩 3-chloromethylbenzothiophene 3216-47-5 C9H7ClS 182.674 —— benzothiophene-2,3-dicarboxylic anhydride 5732-24-1 C10H4O3S 204.206 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 苯并噻吩-3-羧酸甲酯 methyl benzothiophene-3-carboxylate 22913-25-3 C10H8O2S 192.238 —— ethyl benzo[b]thiophene-3-carboxylate 19156-49-1 C11H10O2S 206.265 —— 6-bromobenzo[b]thiophene-3-carboxylic acid 19075-61-7 C9H5BrO2S 257.107 5-溴苯并[B]噻吩-3-甲酸 5-bromobenzo[b]thiophene-3-carboxylic acid 7312-24-5 C9H5BrO2S 257.107 —— pent-4-enyl benzo[b]thiophene-3-carboxylate 1160843-12-8 C14H14O2S 246.33 —— methyl 6-bromobenzo[b]thiophene-3-carboxylate 946428-00-8 C10H7BrO2S 271.134 —— 2-methylbenzo[b]thiophene-3-carboxylic acid 17375-82-5 C10H8O2S 192.238 3-甲醛苯并噻吩 benzothiophene-3-carboxaldehyde 5381-20-4 C9H6OS 162.212 3-羟甲基苯并噻吩 benzo[b]thiophen-3-yl methanol 5381-24-8 C9H8OS 164.228 —— 6-nitro-benzo[b]thiophene-3-carboxylic acid 24964-26-9 C9H5NO4S 223.209 —— 5-nitro-benzo[b]thiophene-3-carboxylic acid 24964-27-0 C9H5NO4S 223.209 苯并[b]噻吩-3-甲酰胺 benzo[b]thiophene-3-carboxamide 858117-17-6 C9H7NOS 177.227 1-苯并噻吩-3-羰酰氯 benzothiophene-3-carbonyl chloride 39827-12-8 C9H5ClOS 196.657 —— N-hydroxy-1-benzothiophene-3-carboxamide 947513-34-0 C9H7NO2S 193.226 —— benzo[b]thiophene-3-carbonyl azide 78676-35-4 C9H5N3OS 203.224 —— bis(benzothiophen-3-yl)methanone —— C17H10OS2 294.398 —— 1-(benzo[b]thiophen-3-yl)-2,2,2-trifluoroethan-1-one 35472-34-5 C10H5F3OS 230.21 3-氨甲基苯并噻吩 3-benzothiophenemethanamine 40615-04-1 C9H9NS 163.243 —— N-phenylbenzo[b]thiophene-3-carboxamide 70608-29-6 C15H11NOS 253.324 —— methyl 3-(benzo[b]thiophen-3-yl)-3-oxopropanoate 250375-51-0 C12H10O3S 234.276 —— 3-(Azidomethyl)-1-benzothiophene 1111881-07-2 C9H7N3S 189.241 —— N-[(2S)-1-hydroxy-3-methylbutan-2-yl]-1-benzothiophene-3-carboxamide 497866-71-4 C14H17NO2S 263.36 - 1
- 2
- 3
反应信息
-
作为反应物:描述:参考文献:名称:无过渡金属脱羧碘化:脱羧氧化交叉偶联的新途径摘要:从廉价且丰富的原材料中构建具有高合成价值的产品非常重要。芳基碘是合成功能性分子的重要组成部分,从化学原料合成芳基碘的有效方法受到高度追捧。在这里,我们报告了一种低成本的脱羧碘化反应,它可以简单地从容易获得的苯甲酸和 I2 中发生。该反应是可扩展的,并且反应的范围和稳健性得到了彻底检查。机理研究表明,该反应不是通过自由基机制进行的,这与经典的 Hunsdiecker 型脱羧卤化反应相反。此外,DFT 研究允许在我们的程序和当前的过渡金属催化脱羧之间进行比较。该程序的实用性在其通过脱羧/C-H 或双脱羧活化(使用 I2 作为末端氧化剂)用于芳烃的氧化交叉偶联中得到证明。该策略允许制备以前无法通过脱羧方法获得的联芳基化合物,并且由于避免了化学计量过渡金属,因此与现有脱羧氧化偶联相比具有其他优势。DOI:10.1021/jacs.7b05155
-
作为产物:描述:参考文献:名称:Bromination, Iodination and Phenylation of Thianaphthenes摘要:DOI:10.1021/ja01139a508
-
作为试剂:描述:1-苯并噻吩-3-羧酸 、 4-[(3-chloro-benzyl)-((R)-2-methyl-azetidine-2-carbonyl)-amino]-butyric acid methyl ester hydrochloride 在 1-苯并噻吩-3-羧酸 作用下, 以87的产率得到4-[[(R)-1-(benzo[b]thiophene-3-carbonyl)-2-methylazetidine-2-carbonyl]-(3-chlorobenzyl)amino]butyric acid methyl ester参考文献:名称:Compounds useful for the treatment of metabolic and inflammatory diseases摘要:本文披露了一些化合物,其分子式如下:这些化合物可以制备为药物组成物,并可用于哺乳动物,包括人类的预防和治疗多种疾病,例如炎症性疾病、传染性疾病、自身免疫性疾病、涉及免疫细胞功能受损的疾病、心血管代谢性疾病和/或增殖性疾病,举例而言。公开号:US08759334B2
文献信息
-
Effects of substituent pattern on the intracellular target of antiproliferative benzo[b]thiophenyl chromone derivatives作者:Yohei Saito、Yukako Taniguchi、Sachika Hirazawa、Yuta Miura、Hiroyuki Tsurimoto、Tomoki Nakayoshi、Akifumi Oda、Ernest Hamel、Katsumi Yamashita、Masuo Goto、Kyoko Nakagawa-GotoDOI:10.1016/j.ejmech.2021.113578日期:2021.10resistant line. A structure-activity relationship study revealed that a 10π-electron system with high aromaticity, juxtaposed 4-oxo and 5-hydroxy groups, and 7-alkoxy groups were important for potent antimitotic activity. Interestingly, two BT-flavonols (3-hydroxyflavone), 16 and 20, with 3-hydroxy and 5-alkoxy groups, induced distinct biological profiles affecting the cell cycle at the G1/S phase by通过用 10π 电子苯并噻吩 (BT) 取代天然黄酮骨架的 6π 选举苯环-B 产生了一种新的生物支架。由于芳环对于配体蛋白质相互作用很重要,因此环 B 的 π 电子系统的这种扩展可能会改变生物活性特征。由此产生的一种受天然产物启发的新型化合物 2-(benzo[ b ]thiophen-3-yl)-5-hydroxy-7-isopropoxy-6-methoxyflavone ( 6 ),有效地阻止了 G2/M 的细胞周期相和 IC 50显示出显着的抗增殖作用0.05–0.08 μM 值对多种人类肿瘤细胞系,包括多药耐药细胞系。构效关系研究表明,具有高芳香性的 10π-电子系统、并列的 4-氧代和 5-羟基以及 7-烷氧基对于有效的抗有丝分裂活性很重要。有趣的是,具有 3-羟基和 5-烷氧基的两种 BT-黄酮醇(3-羟基黄酮)16和20通过与拓扑异构酶相互作用抑制 DNA 复制,诱导了影响
-
[EN] ARYL-1,3-AZOLE DERIVATIVES AND METHODS FOR INHIBITING HEPARNASE ACTIVITY<br/>[FR] DERIVES D'ARYL-1,3-AZOLE ET PROCEDES PERMETTANT D'INHIBER L'ACTIVITE DE L'HEPARANASE申请人:IMCLONE SYSTEMS INC公开号:WO2005030206A1公开(公告)日:2005-04-07The present invention encompasses heparanase inhibitors, particularly to certain 2-substituted heteroaryl-fused and aryl-fused carbazole derivatives that inhibit heparanase, pharmaceutical compositions that contain the compounds, methods for making the compounds, and methods of treating heparanase-dependent diseases and conditions in mammals by administering a therapeutically effective amount of the compounds to the mammals.
-
Piperazine and homopiperazine compounds
-
Palladium-Catalyzed Direct Decarbonylative Phosphorylation of Benzoic Acids with P(O)-H Compounds作者:Ji-Shu Zhang、Tieqiao Chen、Li-Biao HanDOI:10.1002/ejoc.201901865日期:2020.3.8A direct decarbonylative phosphorylation of benzoic acids catalyzed by palladium was disclosed. Under the reaction conditions, a wide range of carboxylic acids coupled readily with all the three kinds of P(O)–H compounds, i.e. secondary phosphine oxides, H‐phosphinates and H‐phosphonates, producing the corresponding organophosphorus compounds in good to high yields. This reaction could be conducted
-
Electrochemical Oxidation Enables Regioselective and Scalable α-C(sp<sup>3</sup>)-H Acyloxylation of Sulfides作者:Huamin Wang、Meng He、Yongli Li、Heng Zhang、Dali Yang、Masanari Nagasaka、Zongchao Lv、Zhipeng Guan、Yangmin Cao、Fengping Gong、Zhilin Zhou、Jingyun Zhu、Supravat Samanta、Abhishek Dutta Chowdhury、Aiwen LeiDOI:10.1021/jacs.1c00288日期:2021.3.10A highly selective, environmentally friendly, and scalable electrochemical protocol for the construction of α-acyloxy sulfides, through the synergistic effect of self-assembly-induced C(sp3)–H/O–H cross-coupling, is reported. It features exceptionally broad substrate scope, high regioselectivity, gram-scale synthesis, construction of complex molecules, and applicability to a variety of nucleophiles
表征谱图
-
氢谱1HNMR
-
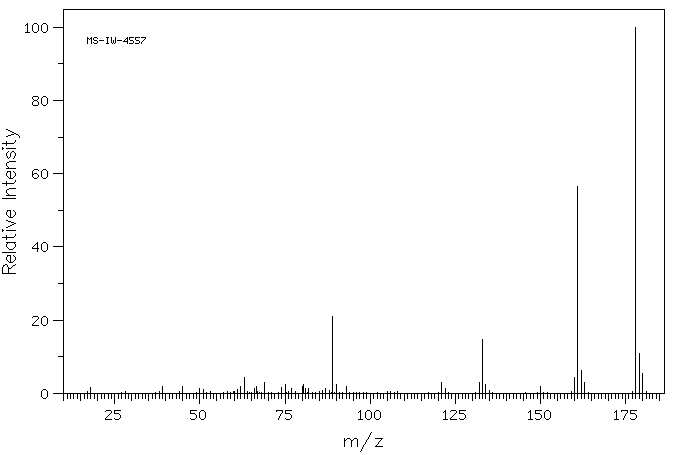
质谱MS
-
碳谱13CNMR
-
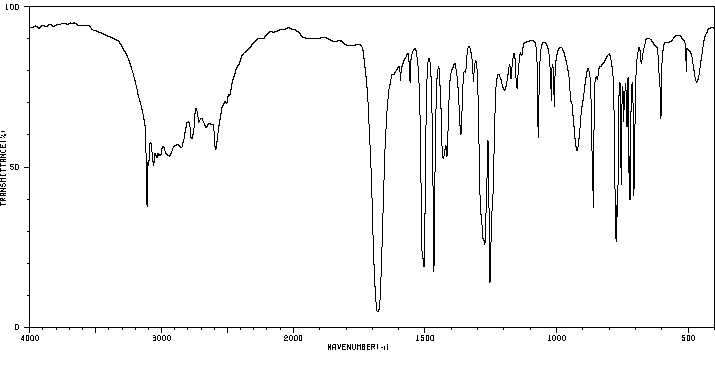
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
齐留通钠
齐留通相关物质A
齐留通亚砜
齐留通-d4
齐留通
雷洛昔芬杂质
邻联甲苯胺砜
试剂4,8-Bis(3,5-dioctyl-2-thienyl)-2,6-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzo[1,2-b:4,5-b']dithiophene
试剂1,1'-[4,8-Bis[4-(2-ethylhexyl)-3,5-difluorophenyl]benzo[1,2-b:4,5-b']dithiophene-2,6-diyl]bis[1,1,1-trimethylstannane]
苯并噻吩-7-醇
苯并噻吩-4-硼酸频哪醇酯
苯并噻吩-3-羧酸甲酯
苯并噻吩-3-硼酸
苯并噻吩-2-羰酰氯
苯并噻吩-2-羧酸肼
苯并噻吩-2-羧酸
苯并噻吩-2-硼酸
苯并噻吩-2-氨基甲酸叔丁酯
苯并噻吩
苯并[c]噻吩
苯并[b]噻吩-7-胺
苯并[b]噻吩-7-羧酸乙酯
苯并[b]噻吩-7-甲醛
苯并[b]噻吩-7-甲腈
苯并[b]噻吩-6-醇
苯并[b]噻吩-6-胺
苯并[b]噻吩-6-羧酸乙酯
苯并[b]噻吩-6-羧酸
苯并[b]噻吩-6-甲腈
苯并[b]噻吩-5-甲腈,2-甲酰基-
苯并[b]噻吩-5-甲磺酰氯
苯并[b]噻吩-4-羧酸甲酯
苯并[b]噻吩-4-羧酸
苯并[b]噻吩-4-甲醛
苯并[b]噻吩-4-甲腈
苯并[b]噻吩-4-基甲醇
苯并[b]噻吩-3-胺盐酸盐
苯并[b]噻吩-3-胺
苯并[b]噻吩-3-羧酸-(2-二烯丙基氨基乙酯)
苯并[b]噻吩-3-硼酸频哪酯
苯并[b]噻吩-3-甲醛肟
苯并[b]噻吩-3-甲酰胺
苯并[b]噻吩-3-基乙酸酯
苯并[b]噻吩-3-乙酸
苯并[b]噻吩-3-乙酰氯
苯并[b]噻吩-3-乙腈
苯并[b]噻吩-2-胺盐酸盐
苯并[b]噻吩-2-羧酸6-氨基-3-氯-甲酯
苯并[b]噻吩-2-羧酸,5-氯-3-(1-甲基乙氧基)-
苯并[b]噻吩-2-羧酸,3-羟基-5-甲氧基-,甲基酯