10-丁基-2-氯-9(10H)-吖啶酮 | 128420-54-2
中文名称
10-丁基-2-氯-9(10H)-吖啶酮
中文别名
——
英文名称
10-n-butyl-2-chloroacridin-9(10H)-one
英文别名
10-Butyl-2-chloroacridin-9-one
CAS
128420-54-2
化学式
C17H16ClNO
mdl
——
分子量
285.773
InChiKey
VYMSWGOFSKMMCE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:443.7±44.0 °C(Predicted)
-
密度:1.210±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):5
-
重原子数:20
-
可旋转键数:3
-
环数:3.0
-
sp3杂化的碳原子比例:0.24
-
拓扑面积:20.3
-
氢给体数:0
-
氢受体数:2
SDS
Section I.Chemical Product and Company Identification
Chemical Name 10-Butyl-2-chloro-9(10H)-acridone
Portland OR
Synonym Not available.
Chemical Formula C17H16ClNO
CAS Number Not available.
Section II. Composition and Information on Ingredients
Chemical Name CAS Number Percent (%) TLV/PEL Toxicology Data
10-Butyl-2-chloro-9(10H)-acridone Not available. Min. 98.0 Not available. Not available.
(GC)
Section III. Hazards Identification
Acute Health Effects No specific information is available in our data base regarding the toxic effects of this material for humans. However,
exposure to any chemical should be kept to a minimum. Skin and eye contact may result in irritation. May be harmful if
inhaled or ingested. Always follow safe industrial hygiene practices and wear proper protective equipment when handling
this compound.
CARCINOGENIC EFFECTS : Not available.
Chronic Health Effects
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY: Not available.
There is no known effect from chronic exposure to this product. Repeated or prolonged exposure to this compound is
not known to aggravate existing medical conditions.
Section IV. First Aid Measures
Eye Contact Check for and remove any contact lenses. DO NOT use an eye ointment. Flush eyes with running water for a minimum
of 15 minutes, occasionally lifting the upper and lower eyelids. Seek medical attention. Treat symptomatically and
supportively.
Skin Contact After contact with skin, wash immediately with plenty of water. Gently and thoroughly wash the contaminated skin with
running water and non-abrasive soap. Be particularly careful to clean folds, crevices, creases and groin. Cover the
irritated skin with an emollient. Seek medical attention. Treat symptomatically and supportively. Wash any contaminated
clothing before reusing.
Inhalation If the victim is not breathing, perform artificial respiration. Loosen tight clothing such as a collar, tie, belt or waistband. If
breathing is difficult, oxygen can be administered. Seek medical attention. Treat symptomatically and supportively.
Ingestion INDUCE VOMITING by sticking finger in throat. Lower the head so that the vomit will not reenter the mouth and throat.
Loosen tight clothing such as a collar, tie, belt, or waistband. If the victim is not breathing, administer artificial respiration.
Examine the lips and mouth to ascertain whether the tissues are damaged, a possible indication that the toxic material
was ingested; the absence of such signs, however, is not conclusive. Seek immediate medical attention and, if possible,
show the chemical label. Treat symptomatically and supportively.
Section V. Fire and Explosion Data
Not available.
Flammability May be combustible at high temperature. Auto-Ignition
Flammable Limits
Flash Points Not available.
Not available.
Combustion Products These products are toxic carbon oxides (CO, CO 2), nitrogen oxides (NO, NO2), halogenated compounds.
WARNING: TOXIC HCl gas produced as a result of combustion.
Fire Hazards
No specific information is available regarding the flammability of this compound in the presence of various materials.
Explosion Hazards Risks of explosion of the product in presence of mechanical impact: Not available.
Risks of explosion of the product in presence of static discharge: Not available.
No additional information is available regarding the risks of explosion.
Fire Fighting Media
SMALL FIRE: Use DRY chemicals, CO 2, water spray or foam.
and Instructions LARGE FIRE: Use water spray, fog or foam. DO NOT use water jet.
Continued on Next Page
10-Butyl-2-chloro-9(10H)-acridone
Section VI. Accidental Release Measures
Spill Cleanup In case of a spill and/or a leak, always shut off any sources of ignition, ventilate the area, and exercise caution. Use a
Instructions shovel to put the material into a convenient waste disposal container. Finish cleaning the spill by rinsing any
contaminated surfaces with copious amounts of water. Consult federal, state, and/or local authorities for assistance on
disposal.
Section VII. Handling and Storage
Handling and Storage Keep away from heat and sources of ignition. Mechanical exhaust required. When not in use, tightly seal the container
and store in a dry, cool place. Avoid excessive heat and light. DO NOT breathe dust. In case of insufficient ventilation,
Information
wear suitable respiratory equipment. If you feel unwell, seek medical attention and show the label when possible. Treat
symptomatically and supportively. Avoid contact with skin and eyes.
Always store away from incompatible compounds such as oxidizing agents.
Section VIII. Exposure Controls/Personal Protection
Engineering Controls Use process enclosures, local exhaust ventilation, or other engineering controls to keep airborne levels below
recommended exposure limits. If user operations generate dust, fume or mist, use ventilation to keep exposure to
airborne contaminants below the exposure limit.
Personal Protection Splash goggles. Lab coat. Dust respirator. Boots. Gloves. A MSHA/NIOSH approved respirator must be used to avoid
inhalation of the product. Suggested protective clothing might not be sufficient; consult a specialist BEFORE handling
this product.
Exposure Limits Not available.
Section IX. Physical and Chemical Properties
Physical state @ 20°C Crystalline powder. Solubility
Not available.
Not available.
Specific Gravity
Molecular Weight 285.77 Partition Coefficient
Not available.
Boiling Point Not available. Vapor Pressure Not available.
Melting Point 119°C (246.2°F) Vapor Density Not available.
Not available. Not available.
Refractive Index Volatility
Critical Temperature Not available. Odor Not available.
Viscosity Not available. Taste Not available.
Section X. Stability and Reactivity Data
Stability
This material is stable if stored under proper conditions. (See Section VII for instructions)
Conditions of Instability
Avoid excessive heat and light.
Incompatibilities
Reactive with oxidizing agents.
Section XI. Toxicological Information
RTECS Number Not available.
Routes of Exposure Eye contact. Ingestion. Inhalation. Skin contact.
Not available.
Toxicity Data
CARCINOGENIC EFFECTS : Not available.
Chronic Toxic Effects
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY: Not available.
There is no known effect from chronic exposure to this product. Repeated or prolonged exposure to this compound is not
known to aggravate existing medical conditions.
Acute Toxic Effects No specific information is available in our data base regarding the toxic effects of this material for humans. However,
exposure to any chemical should be kept to a minimum. Skin and eye contact may result in irritation. May be harmful if
inhaled or ingested. Always follow safe industrial hygiene practices and wear proper protective equipment when handling
this compound.
Continued on Next Page
10-Butyl-2-chloro-9(10H)-acridone
Section XII. Ecological Information
Ecotoxicity Not available.
Environmental Fate Not available.
Section XIII. Disposal Considerations
Recycle to process, if possible. Consult your local or regional authorities. You may be able to dissolve or mix material with
Waste Disposal
a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system. Observe all
federal, state, and local regulations when disposing of this substance.
Section XIV. Transport Information
DOT Classification Not a DOT controlled material (United States).
PIN Number Not applicable.
Proper Shipping Name
Not applicable.
Packing Group (PG) Not applicable.
DOT Pictograms
Section XV. Other Regulatory Information and Pictograms
TSCA Chemical Inventory This product is NOT on the EPA Toxic Substances Control Act (TSCA) inventory. The following notices are required by 40
CFR 720.36 (C) for those products not on the inventory list:
(EPA)
(i) These products are supplied solely for use in research and development by or under the supervision of a technically
qualified individual as defined in 40 CFR 720.0 et sec.
(ii) The health risks of these products have not been fully determined. Any information that is or becomes available will be
supplied on an MSDS sheet.
WHMIS Classification Not available.
(Canada)
EINECS Number (EEC) Not available.
EEC Risk Statements Not available.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
反应信息
-
作为反应物:描述:10-丁基-2-氯-9(10H)-吖啶酮 在 2-二叔丁基膦-2′,4′,6′-三异丙基-3,6-二甲氧基-1,1′-联苯 、 C44H62NO5PPdS 、 水 、 potassium hydroxide 作用下, 以 1,4-二氧六环 为溶剂, 反应 18.0h, 以98%的产率得到10-n-butyl-2-hydroxyacridin-9(10H)-one参考文献:名称:Palladium-Catalyzed Hydroxylation of Aryl and Heteroaryl Halides Enabled by the Use of a Palladacycle Precatalyst摘要:A method for the hydroxylation of aryl and heteroaryl halides, promoted by a catalyst based on a biarylphosphine ligand tBuBrettPhos (1,5) and its corresponding palladium precatalyst (1), is described. The reactions allow the cross-coupling of both potassium and cesium hydroxides with (hetero)aryl halides to afford a variety of phenols and hydroxylated heteroarenes in high to excellent yield.DOI:10.1021/jo500662s
文献信息
-
PHOTOSENSITIVE RESIN COMPOSITION, OXIME SULFONATE COMPOUND, METHOD FOR FORMING CURED FILM, CURED FILM, ORGANIC EL DISPLAY DEVICE, AND LIQUID CRYSTAL DISPLAY DEVICE申请人:FUJIFILM Corporation公开号:US20130171415A1公开(公告)日:2013-07-04Disclosed is a photosensitive resin composition comprising: (Component A) an oxime sulfonate compound represented by Formula (1); (Component B) a resin comprising a constituent unit having an acid-decomposable group that is decomposed by an acid to form a carboxyl group or a phenolic hydroxy group; and (Component C) a solvent wherein in Formula (1) R 1 denotes an alkyl group, an aryl group, or a heteroaryl group, each R 2 independently denotes a hydrogen atom, an alkyl group, an aryl group, or a halogen atom, Ar 1 denotes an o-arylene group or an o-heteroarylene group, X denotes O or S, and n denotes 1 or 2, provided that of two or more R 2 s present in the compound, at least one denotes an alkyl group, an aryl group, or a halogen atom.
-
Mild and General Palladium-Catalyzed Synthesis of Methyl Aryl Ethers Enabled by the Use of a Palladacycle Precatalyst作者:Chi Wai Cheung、Stephen L. BuchwaldDOI:10.1021/ol401796v日期:2013.8.2A general method for the Pd-catalyzed coupling of methanol with (hetero)aryl halides is described. The reactions proceed under mild conditions with a wide range of aryl and heteroaryl halides to give methyl aryl ethers in high yield.描述了甲醇与(杂)芳基卤化物的 Pd 催化偶联的一般方法。该反应在温和条件下进行,使用范围广泛的芳基和杂芳基卤化物,以高产率得到甲基芳基醚。
-
Rational Ligand Design for the Arylation of Hindered Primary Amines Guided by Reaction Progress Kinetic Analysis作者:Paula Ruiz-Castillo、Donna G. Blackmond、Stephen L. BuchwaldDOI:10.1021/ja512903g日期:2015.3.4We report the Pd-catalyzed arylation of very hindered α,α,α-trisubstituted primary amines. Kinetics-based mechanistic analysis and rational design have led to the development of two biarylphosphine ligands that allow the transformation to proceed with excellent efficiency. The process was effective in coupling a wide range of functionalized aryl and heteroaryl halides under mild conditions.
-
POSITIVE PHOTOSENSITIVE RESIN COMPOSITION, METHOD FOR FORMING CURED FILM, CURED FILM, ORGANIC EL DISPLAY DEVICE AND LIQUID CRYSTAL DISPLAY DEVICE申请人:ISHIJI Youhei公开号:US20120045616A1公开(公告)日:2012-02-23A positive photosensitive resin composition including: a resin comprising a structural unit having an acid dissociative group and a structural unit having a functional group capable of forming a covalent bond by reacting with a carboxyl group or a phenolic hydroxyl group; and an acid generator represented by the following formula (I): wherein in formula (I), R 1 represents a hydrogen atom, an alkyl group, an alkenyl group, a cyano group, an aryl group or the like; R 2 represents an alkyl group or an aryl group; each of R 3 and R 4 independently represents a hydrogen atom, an alkyl group an aryl group or the like; and X represents —O—, —S—, —NH— or the like.
-
Mit Silikonen kompatible Photoinitiatoren und diese enthaltende lichtempfindliche Gemische申请人:HOECHST AKTIENGESELLSCHAFT公开号:EP0705865A1公开(公告)日:1996-04-10Es werden Verbindungen der allgemeinen Formel I (SIL - X-)mIN (I), worin SILein Rest der Formel Si(R¹)(R²)(R³) wobei R¹ein Alkyl-, Halogenalkyl- oder Alkoxyrest mit 1 bis 8 Kohlenstoffatomen, ein Alkenylrest, ein Alkenyloxy- oder Acyloxyrest mit 2 bis 8 Kohlenstoffatomen, ein Aryl- oder Aryloxyrest mit 6 bis 10 Kohlenstoffatomen, ein Dialkyl-, Diaryl- oder Alkylaryl-methylenaminooxyrest mit C₁-C₄-Alkyl- oder C₆-Arylgruppen ist und R² und R³gleiche oder verschiedene Reste der Bedeutung von R¹ oder X - IN sind XCnH2n, INder Rest einer als Photoinitiator bzw. Photosensibilisator wirksamen Verbindung mit mindestens einer an einem aromatischen Kern stehenden Carbonylgruppe, meine Zahl von 1 bis 4 und neine Zahl von 2 bis 12 bedeutet, oder der allgemeinen Formel II SioOo-1 (-X-IN)pR⁴2o+2-p (II) vorgeschlagen, worin R⁴ein Rest der Bedeutung von R¹ ist und mehrere R⁴ untereinander gleich oder verschieden sein können, oeine Zahl von 2 bis 20000 und peine Zahl von 1 bis o ist, und die Symbole X und IN die vorstehend angegebene Bedeutung haben, wobei die Gruppe X an ein zur Carbonylgruppe von IN orthoständiges aromatisches Kohlenstoffatom gebunden ist. Die Verbindungen werden durch Umsetzen einer Verbindung IN - H mit einer Verbindung SIL - X' oder einer Verbindung SioOo-1 (-X')pR⁴2o+2-p in Gegenwart einer Rutheniumverbindung hergestellt. Sie sind als radikalbildende Photoinitiatoren oder als Photosensibilisatoren in Silikonen enthaltenden photopolymerisierbaren Gemischen, insbesondere für die Herstellung wasserlos druckender Flachdruckplatten, geeignet.通式 I 的化合物 (SIL - X-)mIN (I)、 其中 SIL 是式 Si(R¹)(R²)(R³)的基团 其中 R¹ 是具有 1 至 8 个碳原子的烷基、卤代烷基或烷氧基,烯基,具有 2 至 8 个碳原子的烯氧基或酰氧基,具有 6 至 10 个碳原子的芳基或芳氧基,具有 C₁-C₄-烷基或 C₆-芳基的二烷基、二芳基或烷基芳基-亚甲基氨基氧基,以及 R²和R³为R¹或X - IN所定义的相同或不同基团 XCnH2n、 IN 是作为光引发剂或光敏剂的化合物的基团,在芳香核上至少有一个羰基、 误为 1 至 4 的数字,以及 n 是 2 至 12 之间的数字、 或通式 II SioOo-1 (-X-IN)pR⁴2o+2-p (II) 建议采用,其中 R⁴ 是 R¹ 的余数,多个 R⁴ 可以相同,也可以不同、 o 是一个从 2 到 20000 的数字,而 p是从 1 到 o 的数字、 符号 X 和 IN 的含义如上、 其中基团 X 与 IN 的羰基正交的芳香碳原子键合。 这些化合物是通过化合物 IN - H 与化合物 SIL - X' 或化合物 SioOo-1 (-X')pR⁴2o+2-p 在钌化合物存在下反应制备的。它们适合用作自由基形成型光引发剂,或用作含有机硅的可光聚合混合物中的光敏剂,尤其适用于生产无水平版印刷板。
表征谱图
-
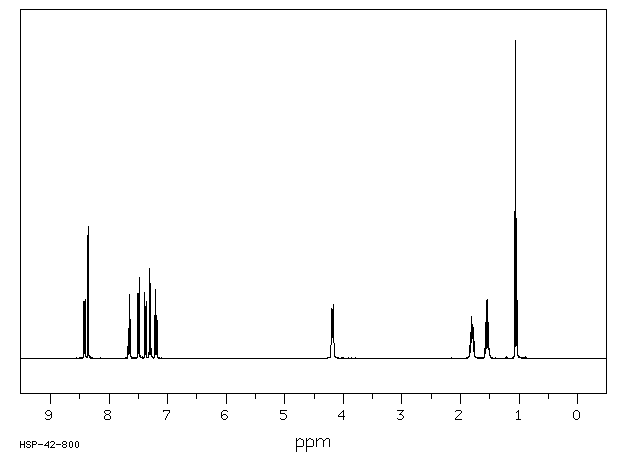
氢谱1HNMR
-
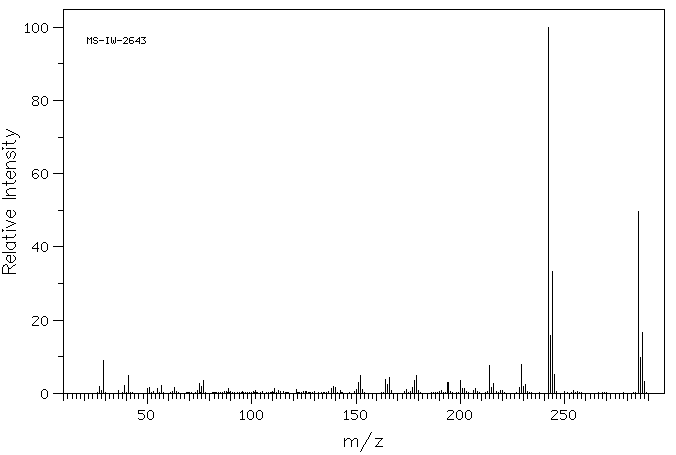
质谱MS
-
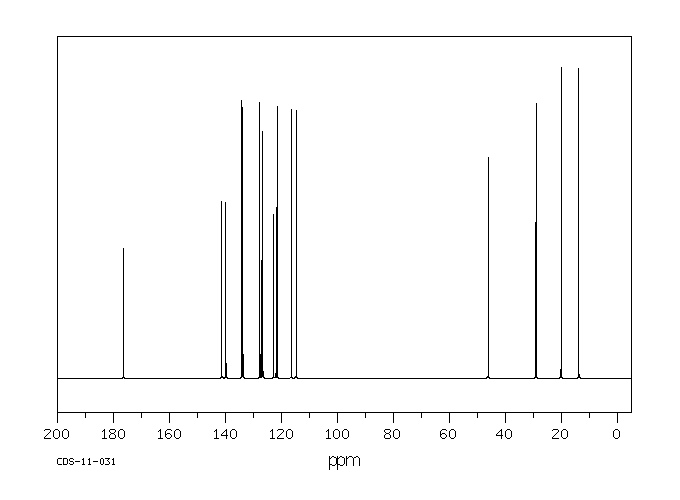
碳谱13CNMR
-
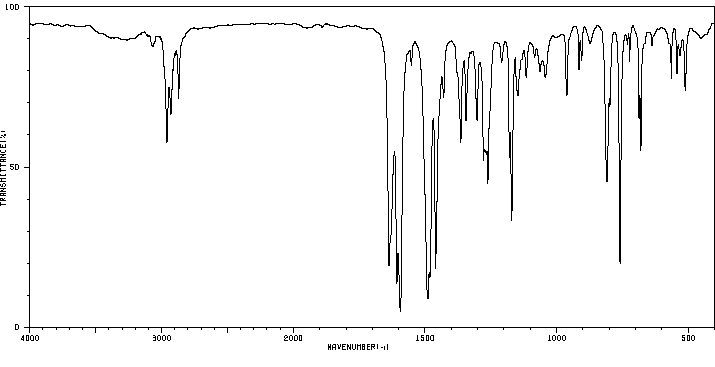
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-4-(叔丁基)-2-(喹啉-2-基)-4,5-二氢噁唑
(SP-4-1)-二氯双(喹啉)-钯
(E)-2-氰基-3-[5-(2,5-二氯苯基)呋喃-2-基]-N-喹啉-8-基丙-2-烯酰胺
(8α,9S)-(+)-9-氨基-七氢呋喃-6''-醇,值90%
(6,7-二甲氧基-4-(3,4,5-三甲氧基苯基)喹啉)
(1-羟基-5-硝基-8-氧代-8,8-dihydroquinolinium)
黄尿酸 8-甲基醚
麻保沙星EP杂质D
麻保沙星EP杂质B
麻保沙星EP杂质A
麦角腈甲磺酸盐
麦角腈
麦角灵
麦皮星酮
麦特氧特
高铁试剂
高氯酸3-苯基[1,3]噻唑并[3,2-f]5-氮杂菲-4-正离子
马波沙星EP杂质F
马波沙星
马来酸茚达特罗杂质
马来酸茚达特罗
马来酸维吖啶
马来酸来那替尼
马来酸四甲基铵
香草木宁碱
颜料红R-122
颜料红210
颜料红
顺式-苯并(f)喹啉-7,8-二醇-9,10-环氧化物
顺式-(alphaR)-N-(4-氯苯基)-4-(6-氟-4-喹啉基)-alpha-甲基环己烷乙酰胺
非那沙星
非那沙星
青花椒碱
青色素863
雷西莫特
隐花青
阿莫地喹-d10
阿莫地喹
阿莫吡喹N-氧化物
阿美帕利
阿米诺喹
阿立哌唑溴代杂质
阿立哌唑杂质B
阿立哌唑杂质38
阿立哌唑杂质1750
阿立哌唑杂质13
阿立哌唑杂质
阿立哌唑杂质
阿尔马尔
阿加曲班杂质43