1H,1H,8H,8H-十二氟-1,8-辛二醇 | 90177-96-1
中文名称
1H,1H,8H,8H-十二氟-1,8-辛二醇
中文别名
2,2,3,3,4,4,5,5,6,6,7,7-十氟-1,8-辛二醇;1,8-二羟基-2,2,3,3,4,4,5,5,6,6,7,7-十二氟辛烷;2,2,3,3,4,4,5,5,6,6,7,7-十二氟-1,8-辛二醇
英文名称
1H,1H,8H,8H-dodecafluoro-1,8-octanediol
英文别名
2,2,3,3,4,4,5,5,6,6,7,7-dodecafluorooctan-1,8-diol;1H,1H,8H,8H-perfluorooctane-1,8-diol;1H,1H,8H,8H-Perfluoro-1,8-octanediol;dodecafluoro-1,8-octanediol;2,2,3,3,4,4,5,5,6,6,7,7-Dodecafluoro-1,8-octanediol;2,2,3,3,4,4,5,5,6,6,7,7-dodecafluorooctane-1,8-diol
CAS
90177-96-1
化学式
C8H6F12O2
mdl
——
分子量
362.115
InChiKey
XZJPYETUABEQFI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:80-83°C
-
沸点:105°C 0,08mm
-
密度:1.636±0.06 g/cm3(Predicted)
-
闪点:105°C/0.08mm
-
溶解度:溶于甲醇。
-
稳定性/保质期:
按规定使用和贮存的情况下,该物质不会分解,并且能够避开氧化物。
计算性质
-
辛醇/水分配系数(LogP):2.8
-
重原子数:22
-
可旋转键数:7
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:40.5
-
氢给体数:2
-
氢受体数:14
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
危险类别码:R36/37/38
-
安全说明:S26,S36/37/39
-
储存条件:存于密闭、阴凉、干燥处。
SDS
2,2,3,3,4,4,5,5,6,6,7,7-Dodecafluoro-1,8-octanediol
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 2,2,3,3,4,4,5,5,6,6,7,7-Dodecafluoro-1,8-octanediol
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Skin corrosion/irritation Category 2
Category 2A
Serious eye damage/eye irritation
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Warning
Hazard statements Causes skin irritation
Causes serious eye irritation
Precautionary statements:
Wash hands thoroughly after handling.
[Prevention]
Wear protective gloves/eye protection/face protection.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
[Response]
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Take off contaminated clothing and wash before reuse.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 2,2,3,3,4,4,5,5,6,6,7,7-Dodecafluoro-1,8-octanediol
Percent: >98.0%(GC)
CAS Number: 90177-96-1
Synonyms: 1,8-Dihydroxy-2,2,3,3,4,4,5,5,6,6,7,7-dodecafluorooctane
C8H6F12O2
Chemical Formula:
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
2,2,3,3,4,4,5,5,6,6,7,7-Dodecafluoro-1,8-
octanediol
Section 4. FIRST AID MEASURES
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Safety glasses. A face-shield, if the situation requires.
Eye protection:
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Crystal- Powder
Form:
Colour: White - Almost white
2,2,3,3,4,4,5,5,6,6,7,7-Dodecafluoro-1,8-
octanediol
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Odour: No data available
pH: No data available
Melting point/freezing point:92°C
Boiling point/range: No data available
Flash point: No data available
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: No data available
Solubility(ies):
[Water] No data available
[Other solvents]
Soluble: Methanol
Section 10. STABILITY AND REACTIVITY
Stable under proper conditions.
Chemical stability:
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Hydrogen fluoride
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
No data available
Crustacea:
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
No data available
Log Pow:
Soil adsorption (Koc): No data available
No data available
Henry's Law
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Does not correspond to the classification standard of the United Nations
Hazards Class:
UN-No: Not listed
2,2,3,3,4,4,5,5,6,6,7,7-Dodecafluoro-1,8-
octanediol
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 2,2,3,3,4,4,5,5,6,6,7,7-Dodecafluoro-1,8-octanediol
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Skin corrosion/irritation Category 2
Category 2A
Serious eye damage/eye irritation
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Warning
Hazard statements Causes skin irritation
Causes serious eye irritation
Precautionary statements:
Wash hands thoroughly after handling.
[Prevention]
Wear protective gloves/eye protection/face protection.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
[Response]
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Take off contaminated clothing and wash before reuse.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 2,2,3,3,4,4,5,5,6,6,7,7-Dodecafluoro-1,8-octanediol
Percent: >98.0%(GC)
CAS Number: 90177-96-1
Synonyms: 1,8-Dihydroxy-2,2,3,3,4,4,5,5,6,6,7,7-dodecafluorooctane
C8H6F12O2
Chemical Formula:
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
2,2,3,3,4,4,5,5,6,6,7,7-Dodecafluoro-1,8-
octanediol
Section 4. FIRST AID MEASURES
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Safety glasses. A face-shield, if the situation requires.
Eye protection:
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Crystal- Powder
Form:
Colour: White - Almost white
2,2,3,3,4,4,5,5,6,6,7,7-Dodecafluoro-1,8-
octanediol
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Odour: No data available
pH: No data available
Melting point/freezing point:92°C
Boiling point/range: No data available
Flash point: No data available
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: No data available
Solubility(ies):
[Water] No data available
[Other solvents]
Soluble: Methanol
Section 10. STABILITY AND REACTIVITY
Stable under proper conditions.
Chemical stability:
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Hydrogen fluoride
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
No data available
Crustacea:
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
No data available
Log Pow:
Soil adsorption (Koc): No data available
No data available
Henry's Law
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Does not correspond to the classification standard of the United Nations
Hazards Class:
UN-No: Not listed
2,2,3,3,4,4,5,5,6,6,7,7-Dodecafluoro-1,8-
octanediol
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
溶于甲醇:几乎无色
反应信息
-
作为反应物:描述:1H,1H,8H,8H-十二氟-1,8-辛二醇 在 sodium meta bi sulfate 、 偶氮二异丁腈 、 sodium hydroxide 作用下, 以 四氢呋喃 、 水 为溶剂, 反应 10.0h, 生成参考文献:名称:氟代烷基烯丙基醚:有用的结构单元,用于合成对环境更安全的氟化多嵌段分子摘要:新颖的三和五嵌段不对称半氟化通式的无支链的醚- [R ˚F CH 2 O(CH 2)3 - [R ' ˚F,含有C≤6全氟化的链,已被合成和表征。在两个步骤中进行合成:全氟烷基碘化物除了氟代烯丙基醚接着还原的C通过用披钯碳多相催化氢化我键。DOI:10.1016/j.jfluchem.2013.08.003
文献信息
-
化合物、液晶组合物、高分子材料和膜申请人:富士胶片株式会社公开号:CN104159996B公开(公告)日:2016-01-20包含下述通式表示的化合物的液晶组合物显示出充分的溶解性,可使用的浓度范围广,显示出优异的雾度降低性[式中,B 1 表示碳原子数为1~40的n价的链状连接基团该链状连接基团包含至少1个-CR 101 R 102 -(R 101 和R 102 为氢原子、卤原子、-COOH、-SO 3 H、-SH、-NH 3 或-OH)且不包含环状结构};L 1 和L 2 表示单键、-O-、-S-、-CO-、-COO-、-OCO-、-COS-、-SCO-、-C(=S)O-、-NR 0 CO-或-CONR 0 -(R 0 表示氢原子或碳原子数为1~6的烷基);A 1 表示2价的芳香族烃基或杂环基;A 2 表示l+1价的芳香族烃基;Sp 1 表示单键或碳原子数为1~10的亚烷基;Hb 1 表示碳原子数为4~30的氟取代烷基,n表示2~6,m表示0~2,l表示2或3。]。
-
Solvent-free catalytic isomerization of perfluoroalkyl allyl ethers作者:Dario Lazzari、Maria Cristina Cassani、Marta Anna Brucka、Gavino Solinas、Marisa PrettoDOI:10.1039/c3nj01300f日期:——A convenient method for the bulk preparation of environmentally safe perfluoroalkyl propen-1-yl ethers via isomerization of primary allyl ethers is presented. Herein the solvent-free isomerization was carried out in the presence of two ruthenium(II) complexes: the ruthenium-hydride [RuClH(CO)(PPh3)3] and the second generation Grubb's type catalyst dichloro[1,3-bis-(2,4,6-trimethylphenyl)-4,5-dimethylimidazol-2-ylidene](thien-2-ylmethylidene)(PCy3) ruthenium(II) known as CatMETium_RF3™. A variety of reaction parameters such as the catalyst loading, the use of a base and UV photoirradiation have been investigated. We found that the presence of Bu3N is beneficial to both catalytic systems and although CatMETium_RF3™ was shown to be more active than [RuClH(CO)(PPh3)3], the catalytic activity of the latter can also be sensibly improved by UV irradiation. Carrying out the isomerization reaction with 0.1 mol% of [RuClH(CO)(PPh3)3] under UV-irradiation led to a ca. 30% improvement of the conversion compared to the same reaction carried out under sunlight and to a 60% improvement when the reaction is carried out in the dark thus mimicking the conditions present in an autoclave.本文介绍了一种通过伯烯丙基醚异构化批量制备环境安全型全氟烷基丙烯-1-基醚的简便方法。这里的无溶剂异构化是在两种钌(II)配合物的存在下进行的:钌酸酐[RuClH(CO)(PPh3)3]和第二代格拉布催化剂二氯[1,3-双(2,4,6-三甲基苯基)-4,5-二甲基咪唑-2-亚基](噻吩-2-亚甲基)(PCy3)钌(II),即 CatMETium_RF3™。我们对催化剂负载、碱的使用和紫外光照射等各种反应参数进行了研究。我们发现,Bu3N 的存在对两种催化体系都有好处,虽然 CatMETium_RF3™ 的活性高于 [RuClH(CO)(PPh3)3],但后者的催化活性也可以通过紫外光照射得到明显改善。在紫外线照射下使用 0.1 摩尔%的[RuClH(CO)(PPh3)3]进行异构化反应,与在阳光下进行的相同反应相比,转化率提高了约 30%,而在黑暗中进行反应时,转化率提高了 60%,从而模拟了高压釜中的条件。
-
FLUOROURETHANE AS AN ADDITIVE IN A PHOTOPOLYMER FORMULATION申请人:Rölle Thomas公开号:US20120231376A1公开(公告)日:2012-09-13The invention relates to a photopolymer formulation comprising matrix polymers, writing monomers, and photoinitiators, to the use of the photopolymer formulation for producing optical elements, in particular for producing holographic elements and images, to a method for illuminating holographic media made of the photopolymer formulation, and to special fluorourethanes.
-
THERMAL ACID GENERATORS AND PHOTORESIST PATTERN TRIMMING COMPOSITIONS AND METHODS申请人:Rohm and Haas Electronic Materials LLC公开号:US20170123313A1公开(公告)日:2017-05-04Provided are ionic thermal acid generators of the following general formula (I): wherein: Ar 1 represents an optionally substituted carbocyclic or heterocyclic aromatic group; W independently represents a group chosen from carboxyl, hydroxy, nitro, cyano, C1-5 alkoxy and formyl; X is a cation; Y independently represents a linking group; Z independently represents a group chosen from hydroxyl, fluorinated alcohols, esters, optionally substituted alkyl, C5 or higher optionally substituted monocyclic, polycyclic, fused polycyclic cycloaliphatic, or aryl, which may optionally comprise a heteroatom, provided at least one occurrence of Z is a hydroxyl group; a is an integer of 0 or greater; b is an integer of 1 or greater; provided that a+b is at least 1 and not greater than the total number of available aromatic carbon atoms of the aromatic group. Also provided are photoresist pattern trimming compositions and methods of trimming a photoresist pattern using the trimming compositions. The thermal acid generators, compositions and methods find particular applicability in the manufacture of semiconductor devices.提供的是以下一般式(I)的离子热酸发生剂: 其中:Ar1代表一个可选取的取代的碳环或杂环芳香基团;W独立地代表从羧基,羟基,硝基,氰基,C1-5烷氧基和甲酰基中选择的一个基团;X是一个阳离子;Y独立地代表一个连接基团;Z独立地代表从羟基,氟化醇,酯类,可选取的取代烷基,C5或更高的可选取的单环,多环,融合多环环烷基或芳基中选择的一个基团,可以可选地包含一个杂原子,但至少有一个Z是一个羟基;a是大于或等于0的整数;b是大于或等于1的整数;但要求a+b至少为1且不大于芳香基团中可用的芳香碳原子的总数。此外,还提供了修剪光刻胶图案的组合物和方法。这些热酸发生剂、组合物和方法在半导体器件制造中具有特殊的适用性。
-
CROSSLINKABLE POLYMERS AND UNDERLAYER COMPOSITIONS申请人:Rohm and Haas Electronic Materials LLC公开号:US20150210793A1公开(公告)日:2015-07-30A crosslinkable polymer comprising: a first unit of the following general formula (I-A) or (I-B): wherein: P is a polymerizable functional group; L is a single bond or an m+1-valent linking group; X 1 is a monovalent electron donating group; X 2 is a divalent electron donating group; Ar 1 and Ar 2 are trivalent and divalent aryl groups, respectively, and carbon atoms of the cyclobutene ring are bonded to adjacent carbon atoms on the same aromatic ring of Ar 1 or Ar 2 ; m and n are each an integer of 1 or more; and each R 1 is independently a monovalent group; and a second unit chosen from general formulae (III) and (IV): wherein R 7 is chosen from hydrogen, fluorine, C 1 -C 3 alkyl and C 1 -C 3 fluoroalkyl, R 8 is chosen from optionally substituted C 1 to C 10 alkyl, and Ar 3 is an optionally substituted aryl group. Underlayer compositions comprise the crosslinkable polymer and a solvent. The crosslinkable polymers and underlayer compositions find particular applicability in the manufacture of semiconductor devices or data storage devices for the formation of high resolution patterns.一种可交联聚合物,包括以下第一单元的一般式(I-A)或(I-B):其中:P是聚合官能团;L是单键或m+1价连接基团;X1是单价电子供体基团;X2是双价电子供体基团;Ar1和Ar2分别是三价和二价芳基基团,环丁烯环上的碳原子与Ar1或Ar2的同一芳环上的相邻碳原子相键合;m和n均为1或更多的整数;每个R1独立地是单价基团;以及从一般式(III)和(IV)中选择的第二单元:其中R7选择自氢、氟、C1-C3烷基和C1-C3氟烷基,R8选择自可选取代的C1至C10烷基,而Ar3是可选取代的芳基基团。底层组成物包括可交联聚合物和溶剂。该可交联聚合物和底层组成物在制造半导体器件或数据存储器件的高分辨率图案形成中具有特定的适用性。
表征谱图
-
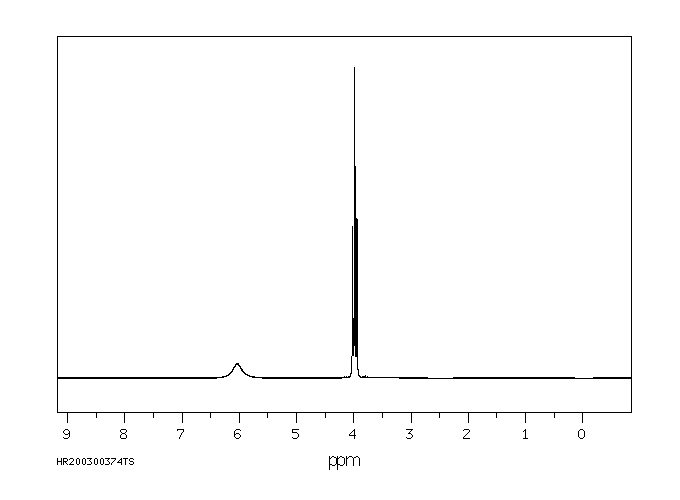
氢谱1HNMR
-
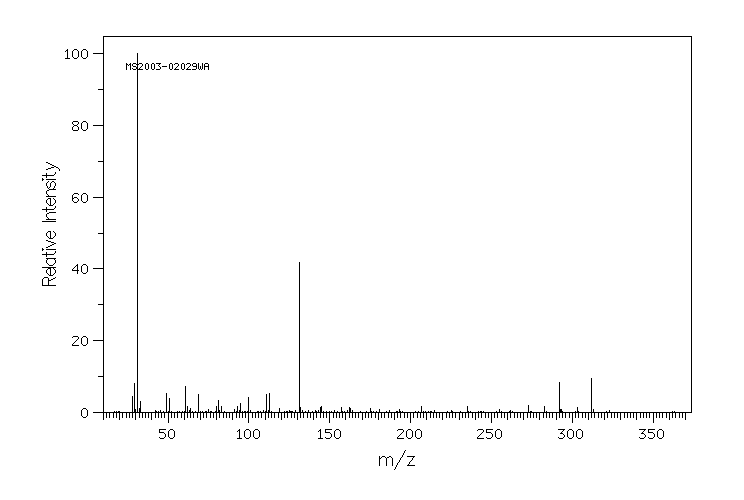
质谱MS
-
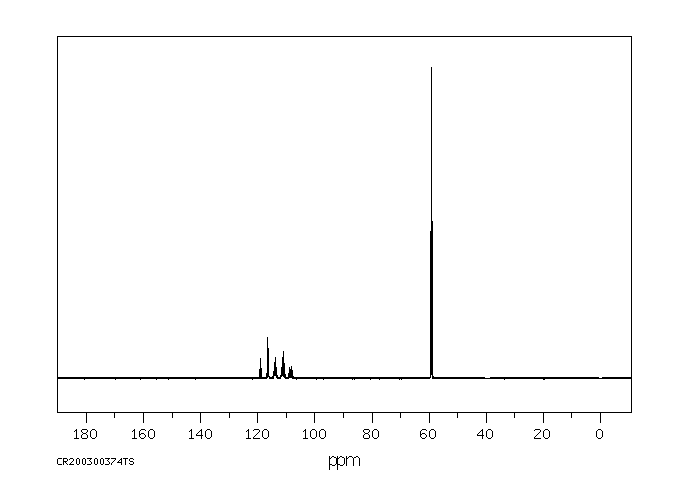
碳谱13CNMR
-
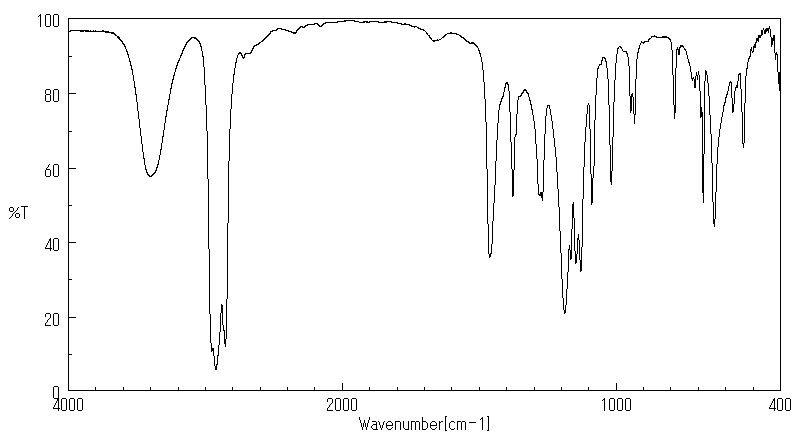
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺-(1S,2S)-1,2-二氢-3-氟邻苯二酚
苏-3-溴-2-丁醇
苏-3-溴-2-丁醇
烯丙基3-氯-2-羟基丙酸酯
溶剂紫36
溴丁醇
水合氯醛
氯醛甜菜碱
氯醛叔丁基半缩醛
氯醛丙基半缩醛
氯二氟乙醛’水合物
氯-(2-氯-3-羟基丙-1-烯基)汞
氘代3-氯-1,2-丙二醇
培氟沙星
四氟乙醇
四氟丙醇
四氟丁二醇
十二氟庚醇
十一氟正己烷-1-醇
六氟异丙醇
六氟丁醇
六氟-1-丙醇
八氟代-1-戊醇
八氟-1,6-己二醇
全氟十醇
全氟-1-辛醇
全氟-1-庚醇
五氟丙醛甲基半缩醛
五氟丙醛水合物
五氟丙醛乙基半缩醛
二溴甘露醇
二氯乙醛水合物
二氯乙氧基合氧钒
二氟乙醛缩半乙醇
乙基3-氟-2-羟基-3-甲基丁酸酯
三溴乙醇
三氟甲基己醇
三氟乙醛缩甲基半醇
三氟乙醛水合物
三氟乙醇
三氟乙基醇-OD
七氟丁醛乙基半缩醛
丁氯醇
rac-2-氯十二烷-1-醇
rac-1-氯十二烷-2-醇
alpha,alpha-二(三氟甲基)-1-氮丙啶甲醇
[2H4]-2-溴-1,3-丙二醇[干冰运输]
[1-氯-3-异丙基氨基-2-丙醇
[1,1-(2)H2]-2-氯乙醇
O-(1,1,3-三氢四氟丙基)-(1-羟基-2,2,2-三氯乙基)甲基膦酸酯