2,2,6,6-四氯环己醇 | 56207-45-5
中文名称
2,2,6,6-四氯环己醇
中文别名
——
英文名称
2,2,6,6-tetrachlorocyclohexanol
英文别名
2,2,6,6-tetrachloro-cyclohexanol;2,2,6,6-Tetrachlor-cyclohexanol;2,2,6,6-tetrachlorocyclohexan-1-ol
CAS
56207-45-5
化学式
C6H8Cl4O
mdl
MFCD00003823
分子量
237.941
InChiKey
RORBTKDJFQCFMD-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:55-60 °C
-
沸点:339.54°C (rough estimate)
-
密度:1.5655 (rough estimate)
-
稳定性/保质期:
遵照规定使用和储存,则不会分解。
计算性质
-
辛醇/水分配系数(LogP):2.8
-
重原子数:11
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
安全信息
-
危险品标志:Xn
-
安全说明:S24/25
-
储存条件:应存于阴凉干燥环境中。
SDS
| Name: | 2 2 6 6-Tetrachlorocyclohexanol 98% Material Safety Data Sheet |
| Synonym: | None Known |
| CAS: | 56207-45-5 |
Synonym:None Known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 56207-45-5 | 2,2,6,6-Tetrachlorocyclohexanol | 98% | 260-055-3 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Runoff from fire control or dilution water may cause pollution.
Extinguishing Media:
In case of fire, use water, dry chemical, chemical foam, or alcohol-resistant foam. Use agent most appropriate to extinguish fire.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 56207-45-5: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: white
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 56.00 - 59.00 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C6H8Cl4O
Molecular Weight: 237.94
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Hydrogen chloride, carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 56207-45-5 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
2,2,6,6-Tetrachlorocyclohexanol - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 56207-45-5: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 56207-45-5 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 56207-45-5 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:描述:参考文献:名称:2,2,6,6-Tetrachlor-cyclohexanon vom Schmp 84� und C6H5O2Cl vom Schmp. 123 bis 124�摘要:DOI:10.1007/bf00904006
-
作为产物:描述:参考文献:名称:Duhamel, Pierre; Leblond, Bertrand; Bidois-Sery, Laure, Journal of the Chemical Society. Perkin transactions I, 1994, # 16, p. 2265 - 2272摘要:DOI:
-
作为试剂:描述:乙醚 在 sodium hydroxide 作用下, 以 2,2,6,6-四氯环己醇 、 水 为溶剂, 以96.5%的产率得到1,3,3-trichloro-7-oxabicyclo[4.1.0]heptane参考文献:名称:Synthesis intermediates containing a hexane ring and processes for their摘要:本发明涉及与以下一般公式(II)对应的新氟代己烷化合物:##STR1## 其中X'.sub.2、X'.sub.3和X'.sub.4相同或不同,并表示卤素或伪卤素,优选为卤素,更优选为氯和氟,条件是当R.sub.1为羟基、氰基、酰胺基、亚甲基、乙氧基、苄氧基、环己氧基和叔丁氧基时,不是所有的卤素同时是氯和R.sub.4、R.sub.3和R.sub.5同时等于H;R.sub.1表示氢、烷基链、烷氧基、环烷基醚、芳香基、芳香基醚或烷氧基、羰基、羧基或酰氧基、氰基、酰胺基、亚甲基或羟基;R.sub.4表示氢、氟原子、烷基链、例如烷基链、芳香基或羰基、羧基或羧酰胺基,或者是通过硫属元素或V族元素,优选为第一周期的元素,例如酰胺基、烷氧基或酰氧基与己烷环相连的基团;其中不同或优选相同的基团R.sub.3和R.sub.5表示氟原子,或优选氢原子,或者也可以是如上所述的烷基链。公开号:US05424460A1
文献信息
-
Nucleophilic fluorination by selective ring opening of α-halooxiranes作者:Pierre Duhamel、Bertrand Leblond、Jean-Marie PoirierDOI:10.1039/c39930000476日期:——Reaction of 1,3,3-trihalo-7-oxabicyclo[4.1.0]heptanes with boron trifluorideâether or HF· pyridine resulted in the regio-and stereo-selective formation in high yield of the corresponding cis-fluorohydrins; using a succession of cyclisations followed by ring-opening reactions by fluoride afforded an iterative preparation of unknown 2,2,6,6-tetrafluorocyclohexanol 13 starting from the chlorinated analogue 1.
-
Process for preparation of halogenated or pseudohalogenated cyclic申请人:Rhone-Poulenc Chimie公开号:US05817890A1公开(公告)日:1998-10-06The present invention relates to new fluorinated hexane compounds corresponding to the following general formula (II): ##STR1## in which X'.sub.2, X'.sub.3, and X'.sub.4 are the same or different, and denote a halogen or a pseudohalogen, preferably a halogen, more preferably chlorine and fluorine, with the condition that, when R.sub.1 is hydroxyl, cyano, amido, imido, ethoxy, benzyloxy, cyclohexyloxy and tert-butoxy, not all the halogens can simultaneously be chlorine and R.sub.4, R.sub.3 and R.sub.5 are simultaneously equal to H; R.sub.1 denotes a hydrogen, a hydrocarbon chain such as an alkyl chain, alkoxy, cycloalkyl ether, an aromatic group, aromatic ether or an alkoxy, carbonyl, carboxyl or acyloxy, cyano, amido, imido or hydroxyl group; R.sub.4 denotes a hydrogen, a fluorine atom, a hydrocarbon chain such as, for example, an alkyl chain, an aromatic group or a carbonyl, carboxyl or carboxamide group or else a radical joined to the hexane ring by a chalcogen or by an element of Group V, preferably of the first Period, such as an amido, alkoxy or acyloxy group; in which the radicals R.sub.3 and R.sub.5, which are different or preferably the same, denote a fluorine, or preferably hydrogen, atom or also a hydrocarbon chain as defined above in the case of R.sub.4.本发明涉及到新的氟代己烷化合物,其对应于以下一般式(II):##STR1## 其中X'.sub.2,X'.sub.3和X'.sub.4相同或不同,表示卤素或伪卤素,优选卤素,更优选氯和氟,条件是当R.sub.1为羟基,氰基,酰胺基,亚胺基,乙氧基,苄氧基,环己氧基和叔丁氧基时,不是所有的卤素都可以同时为氯,而R.sub.4,R.sub.3和R.sub.5同时等于H; R.sub.1表示氢,烃链,例如烷基链,烷氧基,环烷醚,芳香基,芳香醚或烷氧基,羰基,羧基或酰氧基,氰基,酰胺基,亚胺基或羟基; R.sub.4表示氢,氟原子,烃链,例如烷基链,芳香基或羰基,羧基或羧酰胺基,或者是通过硫属或V族元素,优选第一周期的元素,例如酰胺基,烷氧基或酰氧基连接到己烷环的基团; 其中不同或优选相同的基团R.sub.3和R.sub.5表示氟原子,或优选氢原子,也可以是如上所述的烃链,如R.sub.4的情况。
-
Selective activation of carbonchlorine bonds by the electrophilic fragment “Cp★Ru+”作者:Deyanira Rondon、Xiao-Dong He、Bruno ChaudretDOI:10.1016/0022-328x(92)80162-q日期:1992.7The "Cp*Ru+" fragment (1) reacts with cyclic chlorinated hydrocarbons C6H11Cl, 1,2-C6H10Cl2, alpha-C6H6Cl6 and 1.2-C6H9(O)Cl to yield [Cp*Ru(eta-6-C6H6)](CF3SO3) (2) in 60, 90, 35 and 90% yield. respectively; with 2,2,6,6-C6H7Cl4OH the reaction depends upon the conditions but in the absence of dihydrogen [Cp*Ru(eta-6-C6H5OH)](CF3SO3) (4) is the major product. indicating a selectivity for C-Cl activation in the presence of C-O bonds; finally a new series of chlorinated clusters [(Cp*Ru)3(mu-Cl)2(mu-X)(mu-3-CY)](CF3SO3)n (X = Cl, Y = H, n = 1; 5; X = CO, Y = H. n = 2: 6; X = CO, Y = Cl, n = 2: 8) is prepared in high yield from reactions of 1 with CH2Cl2 or 1,2-C6H10Cl(OH).
-
Activation of carbon–sulfur and carbon–chlorine bonds by the electrophilic ruthenium fragment ‘Ru(η-C<sub>5</sub>Me<sub>5</sub>)<sup>+</sup>’作者:Deyanira Rondon、Josianne Delbeau、Xiao-Dong He、Sylviane Sabo-Etienne、Bruno ChaudretDOI:10.1039/dt9940001895日期:——The 'Ru(eta-C5Me5)+' fragment generated by protonation of [Ru(eta-C5Me5)(OMe)}2] by CF3SO3H, reacted with cyclohexene sulfide to give [Ru(eta-C5Me5)(eta6-C6H6)]+ 1 and H2S. With 1,4-dithiane no C-S bond activation was observed but instead the successive formation of [Ru(eta-C5Me5)(S2C4H8)(CF3SO3)] 2 and [Ru(S2C4H8)3]2+ 3, whereas with 1,3-dithiane two compounds resulting from the sequential activation of C-S bonds were isolated as CF3SO3- salts, namely [Ru(eta-C5Me5)SMe)SCH2CH=CH2)}2]2+ 4 and [Ru[C5Me4CH2S(CH2)3SMe]}2]2+ 5. The fragment also reacted with neat dichloromethane to give two trinuclear clusters: [Ru(eta-C5Me5)}3(mu-Cl)3(mu3-CH)]+ 6 and [Ru(C5Me5)}3(mu-Cl)2(mu-CO)(mu3-CH)]2+ 7 in 60 and 30% yield respectively. Its reaction with chlorocyclohexane, 1,2-dichlorocyclohexane and 1,2,3,4,5,6-hexachlorocyclohexane (lindane) yielded 1 and various amounts of H-2 and HCl. In the case of lindane the conversion was only 30% and yielded a 9:1 mixture of 1 and [Ru(eta-C5Me5)(eta6-C6H5Cl)]+ 9. Finally the reaction of C-Cl versus C-O bond activation was compared using 2-chlorocyclohexanone, 2-chlorocyclohexanol and 2,2,6,6-tetrachlorocyclohexanol. It was found that in all cases the C-Cl activation was easier. The first reaction yielded 1, the second the new trinuclear cluster [Ru(C5Me5)}3(mu-Cl)2(mu-CO)(mu3-CCl)]2+ 11 similar to 7. The latter reaction depended upon the reaction conditions, but in tetrahydrofuran at 80-degrees-C, an 80% conversion was observed yielding a 1:7:<1 mixture of 1, [Ru(eta-C5Me5)(eta6-C6H5OH)]+ 10 and [Ru(eta-C5Me5)(eta6-CH5Cl)]+ 9 thus demonstrating a high selectivity for C-Cl activation in the presence of C-O bonds.
-
Hassel; Lunde, Acta Chemica Scandinavica (1947), 1950, vol. 4, p. 200,202作者:Hassel、LundeDOI:——日期:——
表征谱图
-
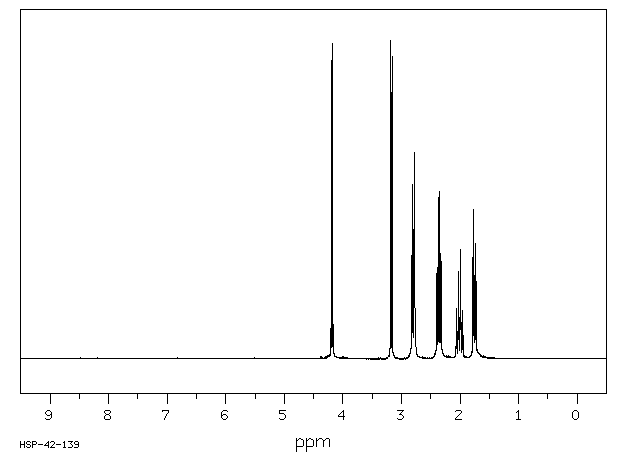
氢谱1HNMR
-
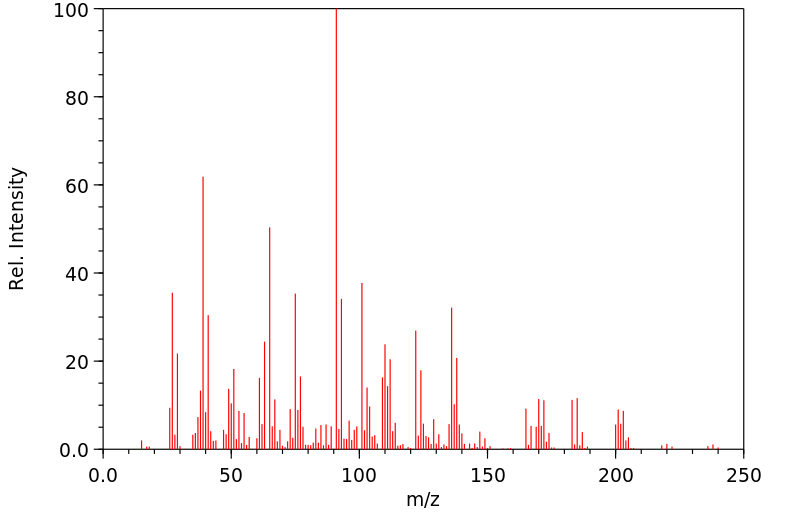
质谱MS
-
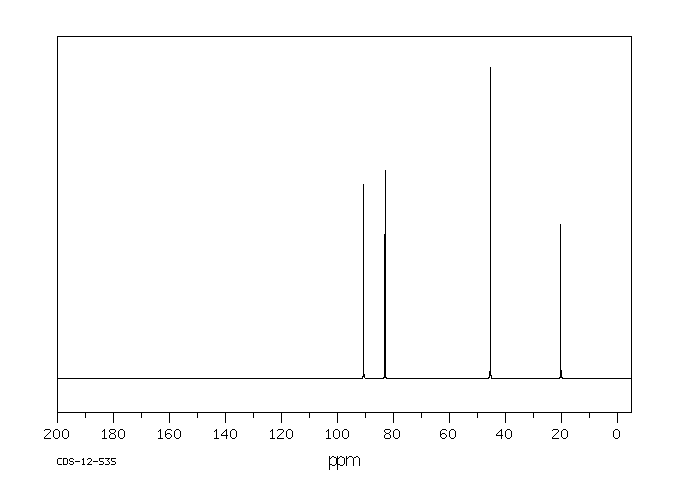
碳谱13CNMR
-
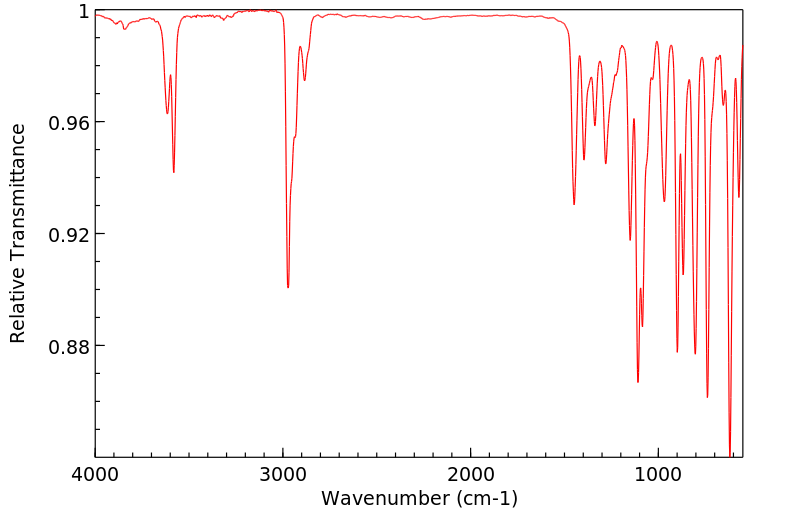
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷