2,4 -二甲基戊二酸 | 2121-67-7
中文名称
2,4 -二甲基戊二酸
中文别名
2,4-二甲基戊二酸
英文名称
2,4-dimethylglutaric acid
英文别名
2,4-dimethylpentanedioic acid
CAS
2121-67-7
化学式
C7H12O4
mdl
MFCD00002660
分子量
160.17
InChiKey
VIWYMIDWAOZEAZ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:104-108 °C
-
沸点:328.8°C (rough estimate)
-
密度:1.3681 (rough estimate)
-
稳定性/保质期:
在常温常压下保持稳定,应避免与不相容的材料及强氧化剂接触。
计算性质
-
辛醇/水分配系数(LogP):0.7
-
重原子数:11
-
可旋转键数:4
-
环数:0.0
-
sp3杂化的碳原子比例:0.714
-
拓扑面积:74.6
-
氢给体数:2
-
氢受体数:4
安全信息
-
安全说明:S24/25
-
海关编码:2917190090
SDS
| Name: | 4-Amino-3 5-Diiodobenzoic Acid 85% Material Safety Data Sheet |
| Synonym: | 3,5-Dijod-4-Aminohippursaeure |
| CAS: | 2121-67-7 |
Synonym:3,5-Dijod-4-Aminohippursaeure
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 2121-67-7 | 2,4-Dimethylglutaric acid, 98+%, mixtu | 218-330-0 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up, then place into a suitable container for disposal. Avoid generating dusty conditions. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 2121-67-7: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Crystalline powder
Color: beige pink
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: > 300 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: H2NC6H2(I)2CO2H
Molecular Weight: 388.92
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Hydrogen chloride, nitrogen oxides, carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 2121-67-7 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
2,4-Dimethylglutaric acid, 98+%, mixture of DL and meso - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 2121-67-7: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 2121-67-7 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 2121-67-7 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2,4-二甲基戊二酸二甲酯 dimethyl 2,4-dimethylpentanedioate 2121-68-8 C9H16O4 188.224 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (2S,4S)-2,4-dimethyl-glutaric acid 107173-20-6 C7H12O4 160.17 (-)-(R,R)-2,4-二甲基戊二酸 (-)-(R,R)-2,4-dimethylglutaric acid 24018-75-5 C7H12O4 160.17 —— (2r,4r)-2,4-Dimethylglutaric acid monomethylester 7586-22-3 C8H14O4 174.197 —— (2S,4S)-2,4-dimethyl-glutaric acid monomethyl ester 100227-54-1 C8H14O4 174.197 2,4-二甲基戊二酸二甲酯 dimethyl 2,4-dimethylpentanedioate 2121-68-8 C9H16O4 188.224 —— 2,4-dimethyl-pentanedioic acid dimethyl ester 4098-66-2 C9H16O4 188.224 —— (+/-)-dimethyl trans-2,4-dimethylglutarate 2121-68-8 C9H16O4 188.224 —— (2S,4S)-methyl 5-hydroxy-2,4-dimethylpentanoate 120850-94-4 C8H16O3 160.213 —— methyl(2R*,4R*)-2,4-dimethyl-5-oxopentanoate 120850-95-5 C8H14O3 158.197 —— racem.-2,4-dimethyl-glutaric acid diethyl ester; 2,4-dimethyl-glutaric acid diethyl ester 21239-22-5 C11H20O4 216.277
反应信息
-
作为反应物:描述:2,4 -二甲基戊二酸 在 乙酸酐 作用下, 反应 6.0h, 以54%的产率得到顺式-3,5-二甲基二氢-2H-吡喃-2,6(3H)-二酮参考文献:名称:碘霉素的形式合成中酸酐的催化烷基化不对称化摘要:献给斯科特教授丹麦在他65之际个生日。 发布时间为致力于斯科特E.丹麦在他65之际特殊部分的部分次生日。 抽象的 用锌试剂对酸酐进行催化脱对称可提供脱氧聚丙酸酯和聚丙酸酯合成子的途径。进行离子霉素的合成,其中使用这种方法将四个片段中的三个组装在一起。两种策略(烯醇硅烷/氧碳鎓偶联和还原环化)均未成功安装C23立体中心,但通过还原/ S N 2方法克服了该立体化学问题。除了合成离子霉素的受保护的非对映异构体之外,C17–C32片段的合成还构成正式的总合成。 用锌试剂对酸酐进行催化脱对称可提供脱氧聚丙酸酯和聚丙酸酯合成子的途径。进行离子霉素的合成,其中使用这种方法将四个片段中的三个组装在一起。两种策略(烯醇硅烷/氧碳鎓偶联和还原环化)均未成功安装C23立体中心,但通过还原/ S N 2方法克服了该立体化学问题。除了合成离子霉素的受保护的非对映异构体之外,C17–C32片段的合成还构成正式的总合成。DOI:10.1055/s-0037-1610108
-
作为产物:描述:参考文献:名称:碘霉素的形式合成中酸酐的催化烷基化不对称化摘要:献给斯科特教授丹麦在他65之际个生日。 发布时间为致力于斯科特E.丹麦在他65之际特殊部分的部分次生日。 抽象的 用锌试剂对酸酐进行催化脱对称可提供脱氧聚丙酸酯和聚丙酸酯合成子的途径。进行离子霉素的合成,其中使用这种方法将四个片段中的三个组装在一起。两种策略(烯醇硅烷/氧碳鎓偶联和还原环化)均未成功安装C23立体中心,但通过还原/ S N 2方法克服了该立体化学问题。除了合成离子霉素的受保护的非对映异构体之外,C17–C32片段的合成还构成正式的总合成。 用锌试剂对酸酐进行催化脱对称可提供脱氧聚丙酸酯和聚丙酸酯合成子的途径。进行离子霉素的合成,其中使用这种方法将四个片段中的三个组装在一起。两种策略(烯醇硅烷/氧碳鎓偶联和还原环化)均未成功安装C23立体中心,但通过还原/ S N 2方法克服了该立体化学问题。除了合成离子霉素的受保护的非对映异构体之外,C17–C32片段的合成还构成正式的总合成。DOI:10.1055/s-0037-1610108
文献信息
-
Photochemical Rearrangement of <i>N</i>-Mesyloxylactams: Stereospecific Formation of <i>N</i>-Heterocycles作者:Alexandre Drouin、Dana K. Winter、Simon Pichette、Samuel Aubert-Nicol、Jean Lessard、Claude SpinoDOI:10.1021/jo101805q日期:2011.1.7N-Mesyloxylactams undergo an efficient ring-contraction to N-heterocycles of various ring sizes. Yields increase with the degree of substitution α to the carbonyl. The stereochemical information of a chiral migrating carbon is conserved making this reaction a synthetically useful complement to the well-known Hofmann, Curtius, Lossen, and Schmidt rearrangements.N-甲氧基内酰胺类对各种环大小的N-杂环进行有效的环收缩。产率随羰基的取代度α而增加。保留了手性迁移碳的立体化学信息,使该反应成为众所周知的霍夫曼,库尔蒂乌斯,洛森和施密特重排的合成有用补充。
-
D-proline derivatives申请人:Hoffmann-La Roche Inc.公开号:US06103910A1公开(公告)日:2000-08-15New compounds have the formula: ##STR1## wherein R, R.sup.1, X and Y have the meanings described herein. Methods are set forth for synthesizing these compounds and using these compounds to treat diseases associated with amyloidosis, such as Alzheimer's disease, maturity onset diabetes mellitus, familial amyloid polyneuropathy, scrapie, and Kreuzfeld-Jacob disease.新化合物的化学式为:##STR1## 其中R、R.sup.1、X和Y的含义如本文所述。本文提供了合成这些化合物和使用这些化合物治疗与淀粉样变性相关的疾病的方法,如阿尔茨海默病、成人起病糖尿病、家族性淀粉样多发性神经病、痱子病和克雅氏病。
-
Regioselective Photochemical Rearrangement of N-Mesyloxylactams作者:Simon Pichette、Samuel Aubert-Nicol、Jean Lessard、Claude SpinoDOI:10.1002/ejoc.201101222日期:2012.3N-Mesyloxylactams can undergo ring contraction either by C-3 (usually observed) or C-5 migration. C-5 migration can occur when the C-3 migration product possesses ring strain, but it does not usually compete with C-3 migration. The greater preference for C-3 migration is due to the carbonyl oxygen atom, which greatly stabilizes the intermediate.N-Mesyloxylactams 可以通过 C-3(通常观察到)或 C-5 迁移进行环收缩。当 C-3 迁移产物具有环应变时会发生 C-5 迁移,但它通常不与 C-3 迁移竞争。C-3 迁移的更大偏好是由于羰基氧原子,它极大地稳定了中间体。
-
FUNCTIONALIZED MONOMERS AND POLYMERS申请人:DiBiase Stephen Augustine公开号:US20120245063A1公开(公告)日:2012-09-27This invention relates to a composition, comprising: an unsaturated functionalized monomer of from about 5 to about 30 carbon atoms, which is: (a) polymerized to form a functionalized polymer; (b) copolymerized with a comonomer to form a functionalized copolymer; or (c) reacted with an enophilic reagent to form a polyfunctionalized monomer. The polyfunctionalized monomer may be polymerized to form a polyfunctionalized polymer which may be further reacted with one or more additional reagents. The invention relates to lubricants, functional fluids, fuels, dispersants, detergents and polymeric resins.这项发明涉及一种组合物,包括:一个由约5至约30个碳原子的不饱和官能化单体组成,该单体:(a)聚合形成官能化聚合物;(b)与共聚单体共聚形成官能化共聚物;或(c)与亲亚烯试剂反应形成多官能单体。多官能单体可以聚合形成多官能聚合物,该聚合物可以进一步与一个或多个额外试剂反应。该发明涉及润滑剂、功能流体、燃料、分散剂、清洁剂和聚合树脂。
-
Enamine chemistry. Part III. Reaction of αβ-unsaturated acid chlorides with enamines of acyclic ketones. Preparation of cyclohexane-1,3-diones作者:J. R. Hargreaves、P. W. Hickmott、B. J. HopkinsDOI:10.1039/j39680002599日期:——isopropyl ketone and the dimethylamine enamine of di-isopropyl ketone gave 2,2,4-trimethyl- and 2,2,4,4-tetramethyl-cyclohexane-1,3-diones respectively. The morpholine enamine of dibenzyl ketone gave 3-morpholino-2,4-diphenyl-cyclohex-2-enone which could not be hydrolysed to the cyclohexane-1,3-dione. The mechanism of the reaction is discussed.
表征谱图
-
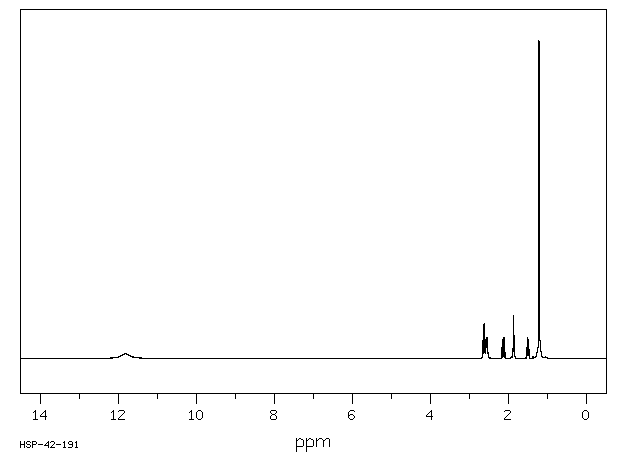
氢谱1HNMR
-
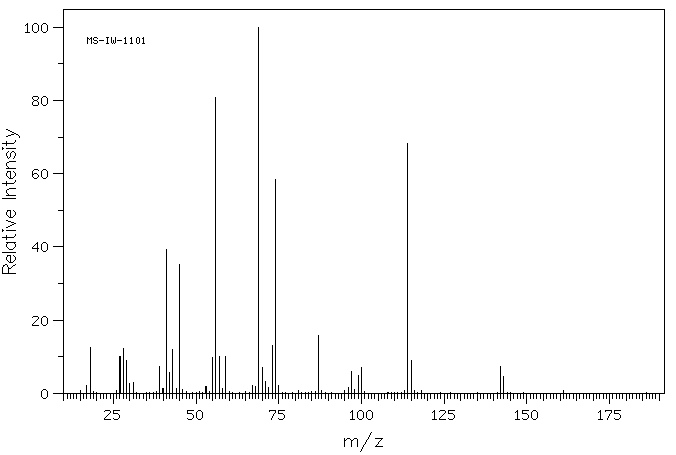
质谱MS
-
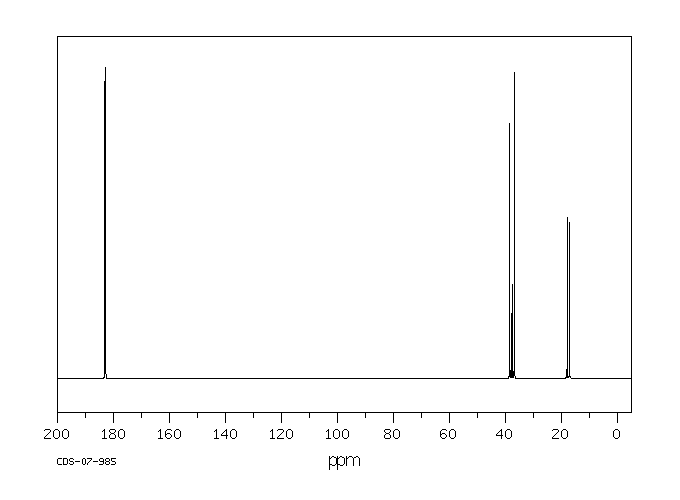
碳谱13CNMR
-
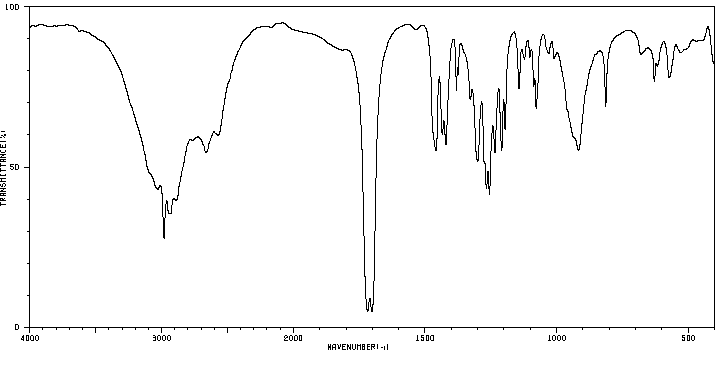
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯