氯甲烷 | 74-87-3
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:−97 °C(lit.)
-
沸点:−24.2 °C(lit.)
-
密度:0.915 g/mL at 25 °C(lit.)
-
蒸气密度:1.74 (vs air)
-
闪点:<-30 °F
-
溶解度:水中的溶解度:5.32g/Lat25°C
-
介电常数:12.6(-20℃)
-
暴露限值:TLV-TWA 50 ppm (~105 mg/m3) (ACGIH), 100 ppm (~210 mg/m3) (OSHA); ceiling 100 ppm (MSHA), 200 ppm (OSHA); TLV STEL 100 ppm (ACGIH); carcinogenicity: Animal Inadequate Evidence, Human Inad equate Evidence (IARC).
-
物理描述:Methyl chloride appears as a colorless gas with a faint sweet odor. Shipped as a liquid under its vapor pressure. A leak may either be liquid or vapor. Contact with the liquid may cause frostbite by evaporative cooling. Easily ignited. Vapors heavier than air. Can asphyxiate by the displacement of air. Under prolonged exposure to fire or intense heat the containers may rupture violently and rocket. Used to make other chemicals and as a herbicide.
-
颜色/状态:Colorless compressed gas or liquid
-
气味:Faint sweet ethereal odor
-
味道:Sweet taste
-
蒸汽密度:1.8 (NTP, 1992) (Relative to Air)
-
蒸汽压力:4300 mm Hg at 25 °C
-
亨利常数:0.01 atm-m3/mole
-
大气OH速率常数:4.36e-14 cm3/molecule*sec
-
稳定性/保质期:
-
自燃温度:632 °C
-
分解:... In contact with moisture undergoes slow decomposition to hydrochloric acid and methanol.
-
粘度:0.00027 Pa.s at 20 °C (liquid, 0.5 MPa)
-
腐蚀性:Methyl chloride will attack some forms of plastics, rubber and coatings.
-
燃烧热:-5290 Btu/lb = -2939 cal/g = -123.1X10+5 J/kg
-
汽化热:18.92 kJ/mol at 25 °C; 21.40 kJ/mol at boiling point
-
表面张力:Liquid Surface Tension: 16.2 dynes/cm = 0.0162 N/m at 20 °C
-
电离电位:11.28 eV
-
气味阈值:Odor recognition in air: 1.00x10+1 ppm (chemically pure)
-
折光率:Index of refraction: 1.3712 at -23.7 °C (liquid)
-
保留指数:332.92;332.92;332;340;332;325.2;332;326;332;332
计算性质
-
辛醇/水分配系数(LogP):0.8
-
重原子数:2
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
ADMET
安全信息
-
危险等级:2.1
-
立即威胁生命和健康浓度:2,000 ppm
-
安全说明:S16,S33,S9
-
危险品运输编号:UN 1993 3
-
WGK Germany:2
-
海关编码:2903110000
-
危险类别:2.1
-
危险类别码:R12
-
RTECS号:PA6300000
-
包装等级:O52
-
危险标志:GHS02,GHS08
-
危险性描述:H220,H280,H351,H361fd,H373
-
危险性防范说明:P210,P281,P410 + P403
-
储存条件:储存注意事项: - 储存在阴凉、通风的有毒气体专用库房。 - 远离火种、热源,库温不宜超过30℃。 - 应与氧化剂分开存放,切忌混储。 - 使用防爆型照明和通风设施,并禁止使用易产生火花的机械设备和工具。 - 储区应配备泄漏应急处理设备。
制备方法与用途
氯甲烷,分子式为CH3Cl。又称甲基氯,是一种无色易液化的气体,具有乙醚的气味和甜味。其相对密度为0.92,熔点-97.6℃,沸点-23.7℃,空气中的比重为1.785,在-23.7℃时折光率为1.3712。微溶于水,易溶于乙醇、氯仿、苯、四氯化碳和冰醋酸等介质中。与空气形成的爆炸性混合物的爆炸极限为8.1%-17.2%(体积)。在高温条件下,它能水解成甲醇和盐酸,并加热或遇火生成光气。此外,氯甲烷还能与金属镁反应生成甲基氯化镁。
应用领域 1. 合成甲基氯硅烷甲基氯硅烷是制备有机硅材料不可或缺的原料。主要合成方法包括格利雅法、有机锂法、缩合法和直接法,其中直接法目前是工业化生产甲基氯硅烷的唯一途径。其工艺流程为:将二氧化硅含量大于99%的硅矿石在1400℃下碳还原冷却得到硅块;再经过破碎、磨粉处理,筛选出一定粒径范围的硅粉;接着让硅粉与氯甲烷直接反应合成甲基氯硅烷。
2. 用作有机合成中的溶剂 用途氯甲烷属于有机卤化物。微溶于水,易溶于氯仿、乙醚、乙醇和丙酮等介质中。它用于生产甲基氯硅烷、四甲基铅以及甲基纤维素等;少量也用于季铵化合物和农药的合成,在异丁橡胶生产过程中作为溶剂使用。此外,氯甲烷还广泛用作溶剂、提取剂、推进剂、制冷剂和局部麻醉剂,并用于农药、医药及香料等行业。
制备 毒性与防治 急性毒性小鼠吸入6小时LC50为6.6克/立方米。职业中毒主要由吸入蒸气引起,症状包括头痛、眩晕、恶心和呕吐等。大鼠急性吸入的半致死浓度(LC50)约为5300毫克/立方米(4小时),口服LD50为1800毫克/公斤。
爆炸物危险特性氯甲烷与空气混合后在明火或受热条件下可能会发生爆炸。燃烧时产生有毒的氯化物烟雾。
可燃性危险特性 储运特性应储存在通风良好、干燥低温的库房中,避免与氧气、空气等助燃气体钢瓶混存。搬运时轻装轻卸。
灭火剂使用雾状水或泡沫进行灭火较为有效。
职业标准时间加权平均容许浓度(TWA)为105毫克/立方米;短时间暴露极限值(STEL)为210毫克/立方米。
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— Methylchlorid-37Cl 1762-22-7 CH3Cl 52.0348
反应信息
-
作为反应物:参考文献:名称:Little, J., British Journal of Industrial Medicine, 1955, vol. 12, p. 304 - 308摘要:DOI:
-
作为产物:参考文献:名称:卤代烷的气相SN2和E2反应摘要:已测量甲基、乙基、正丙基、异丙基、叔丁基和新戊基氯化物和溴化物与以下亲核试剂的气相反应的速率系数,按碱度递减的顺序列出:HO - 、CH 3 O - 、F - 、HO - (H 2 O)、CF 3 CH 2 O - 、H 2 NS - 、C 2 F 5 CH 2 O - 、HS - 和Cl - 。对于氯甲烷,反应效率首先显着低于 1,以 HO - (H 2 O) 作为亲核试剂,而对于甲基溴,以 HS - 作为亲核试剂;在这两种情况下,总反应放热度约为 30 kcal mol -1 。这些卤化物与强碱反应缓慢的早期结论被证明是错误的。在速率较慢的区域,氧阴离子通过消除与烷基氯化物和溴化物反应,而相同碱度的硫阴离子通过取代反应。这种差异是由于硫碱的消除速度减慢;与相同碱度的氧阴离子相比,硫阴离子没有增加亲核性DOI:10.1021/ja00180a003
-
作为试剂:参考文献:名称:NOVEL CATALYTIC METHOD FOR THE PRODUCTION OF FLUOROALKYLENES FROM CHLOROFLUOROHYDROCARBONS摘要:生产公式为R—CF═CHR的产品的方法: 其中R为F或CF3,R1在R为F时为F,在R为CF3时为H, 通过在适当催化剂的存在下,将公式为CF3—R2的反应物与选择自的还原剂(甲烷、氯化甲基或二者的混合物)在气相反应中反应。公开号:US20060217577A1
文献信息
-
수율이 개선된 무수당 알코올의 디에테르 제조 방법申请人:SAMYANG CORPORATION 주식회사 삼양사(120110515934) Corp. No ▼ 110111-4720945BRN ▼101-86-66838公开号:KR102152693B1公开(公告)日:2020-09-08본 발명은 무수당 알코올의 디에테르를 제조하는 방법에 관한 것으로, 더욱 상세하게는, 무수당 알코올과 알킬화 시약(예컨대, 디메틸 설페이트 또는 메틸 클로라이드)을 반응시켜 무수당 알코올의 디에테르를 제조함에 있어서, 무수당 알코올의 모노에테르를 무수당 알코올과 함께 알킬화 시약과 반응시킴으로써, 무수당 알코올을 단독으로 알킬화 시약과 반응시킨 경우에 비하여 수율을 향상시킬 수 있고, 또한 상기 무수당 알코올의 모노에테르의 공급원으로서 무수당 알코올의 디에테르 정제시 발생하는 증류 부산물을 활용할 수 있어 공정 폐기물을 줄일 수 있고, 공정의 원가를 절감할 수 있는 방법에 관한 것이다.
-
Orthocarbonsäure-ester mit 2,4,10-Trioxaadamantanstruktur als Carboxylschutzgruppe; Verwendung zur Synthese von substituierten Carbonsäuren mit Hilfe von<i>Grignard</i>-Reagenzien
-
一种用于制备肟菌酯的中间体及其合成方法
-
Intermediates useful for the preparation of antihistaminic piperidine derivatives申请人:Merrell Pharmaceuticals, Inc.公开号:US06348597B2公开(公告)日:2002-02-19The present invention is related to a novel intermediates and processes which are useful in the preparation of certain antihistaminic piperidine derivatives of the formula wherein W represents —C(═O)— or —CH(OH)—; R1 represents hydrogen or hydroxy; R2 represents hydrogen; R1 and R2 taken together form a second bond between the carbon atoms bearing R1 and R2; n is an integer of from 1 to 5; m is an integer 0 or 1; R3 is —COOH or —COOalkyl wherein the alkyl moiety has from 1 to 6 carbon atoms and is straight or branched each of A is hydrogen or hydroxy; and pharmaceutically acceptable salts and individual optical isomers thereof, with the proviso that where R1 and R2 are taken together to form a second bond between the carbon atoms bearing R1 and R2 or where R1 represented hydroxy, m is an integer 0.
-
Thermal properties of H2SnCl6 complexes作者:Tadeusz Janiak、Jerzy BłażejowskiDOI:10.1016/0040-6031(89)87045-5日期:1989.3Russell-Jones theory for the dissociative volatilization process and a standard approach based on the Arrhenius model. The values of the parameters characterizing the thermal properties of alkanaminium hexachlorostannates, i.e. temperatures of the thermal effects, and the thermochemical and kinetic constants of thermolysis, depend on the number, length and structure of the alkyl substituent. The essential摘要 热分析方法(DTA、TG 和 DTG)用于研究具有通式 [(CnH2n+1)pNH4-p]2SnCl6(其中 n = 1–4 和 p = 2–4)的无支链复合盐的热行为和其他几种环状和开链支链脂肪族链烷胺六氯锡酸盐。这些化合物的热解离通常可以使用等式来概括,其中 A 表示烷基(a = 0 和 s = 1 表示季铵盐,a = 1 和 s = 0 表示其他研究的化合物)。具有简单结构的衍生物的热解发生在一个步骤中并导致它们的完全挥发。其他化合物的分解,通常具有复杂的结构,伴随着副反应。实验 TG 曲线用于检查热解的热力学和动力学。热解离焓基于范特霍夫方程进行评估。导出的值与可用的文献数据一起用于确定盐的形成焓和晶格能。后者的数量也使用 Kapustinskii-Yatsimirskii 公式进行了检查。使用Jacobs 和Russell-Jones 解离挥发过程理论和基于Arrhenius
表征谱图
-
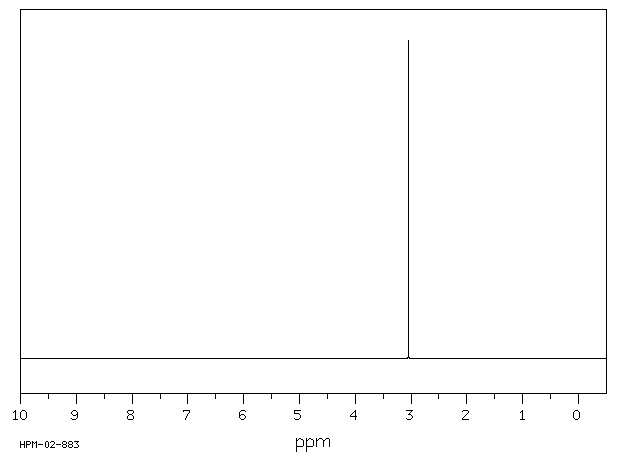
氢谱1HNMR
-
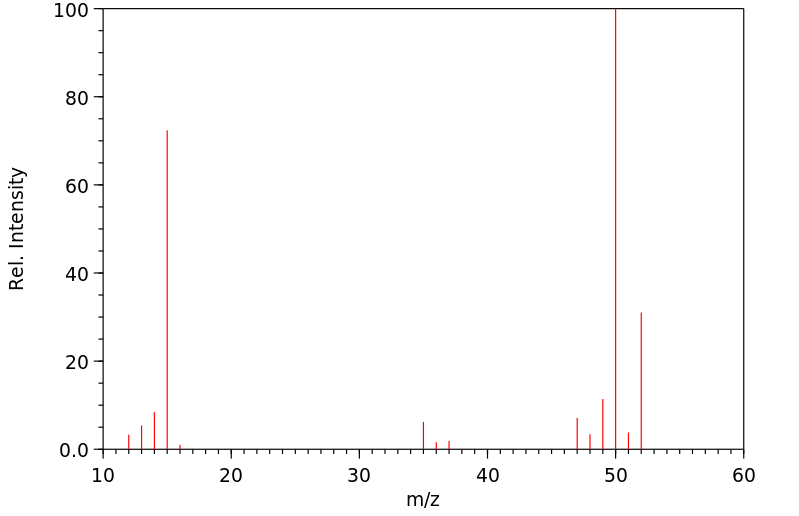
质谱MS
-
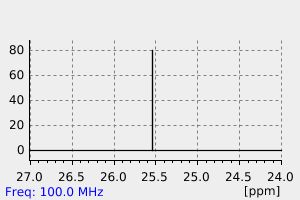
碳谱13CNMR
-
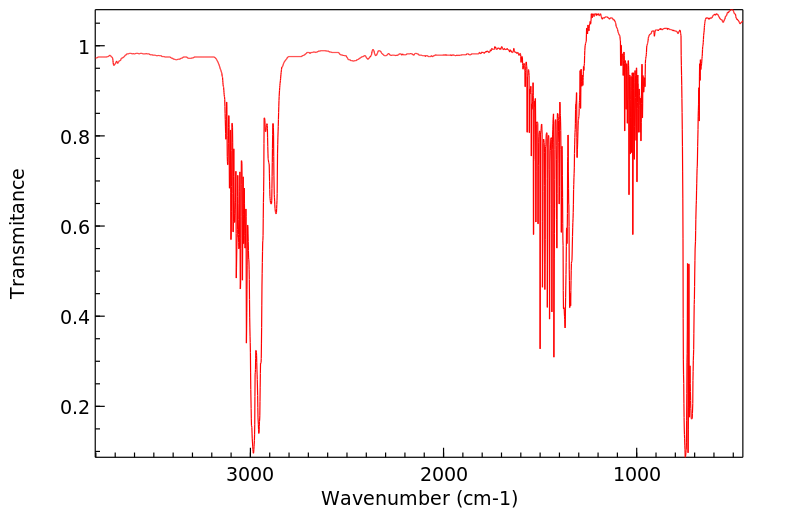
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息